Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2017
Table of contents
- Executive summary
- 1 Background
- 2 Report overview
-
3 Regulatory program for the use of nuclear substances
- 3.1 CNSC regulatory effort
- 3.2 Licensing
- 3.3 Certification of prescribed equipment
- 3.4 Certification of exposure device operators
- 3.5 Certification of Class II radiation safety officers
- 3.6 RSO appointment for nuclear substances and radiation devices licences
- 3.7 RSO appointment for waste nuclear substance licences
- 3.8 Licensing and certification decisions
- 3.9 Compliance verification and enforcement
- 3.10 Stakeholder engagement
- 3.11 International commitments
- 4 Regulatory developments
- 5 Safety performance – all sectors combined
- 6 Medical sector
- 7 Industrial sector
- 8 Academic and research sector
- 9 Commercial sector
- 10 Waste nuclear substance sector
- 11 Conclusion
- Appendix A: Radiation exposure
- Appendix B: Enforcement actions issued in 2017
- Appendix C: List of reported events in 2017
- Appendix D: Inspections conducted in 2017
- Appendix E: Compliance rating levels
- Appendix F: Grading inspections
- Appendix G: Relevant Regulatory References
Executive summary
The Regulatory Oversight Report on the Use of Nuclear Substances in Canada: 2017 summarizes the safety performance of 1,590 licensees, which hold a total of 2,191 licences, and which are authorized by the Canadian Nuclear Safety Commission (CNSC) for the use of nuclear substances and prescribed equipment in the medical, industrial, academic and research, commercial, and waste nuclear substance sectors. This is the first time the waste nuclear substance licensees have been included in this regulatory oversight report.
The CNSC regulates the nuclear industry in Canada through a comprehensive program of licensing, certification, compliance verification, enforcement and reporting. For each sector described in this report, CNSC staff evaluate safety performance through inspections, assessments, and reviews of licensee programs and processes.
CNSC staff use a well-established safety and control area (SCA) framework in evaluating each licensee’s safety performance. The framework includes 14 SCAs covering all technical areas of regulatory oversight. For the purpose of this report, safety performance is evaluated by presenting licensees’ regulatory compliance in select SCAs (i.e., management system, operating performance, radiation protection, security and ‒ for the waste nuclear substance sector only ‒environmental protection), as well as effective doses to workers and reported events.
In 2017, as part of the ongoing regulatory oversight of licensees, CNSC staff conducted compliance verification activities consisting of field inspections, desktop reviews and technical assessments of licensee activities. The evaluations of findings for the SCAs covered in this report show that, overall, licensees made adequate provision for the protection of the health, safety, national security,and the environment from the use of nuclear substances, and took the measures required to implement Canada’s international obligations. Based on these evaluations, CNSC staff conclude that the use of nuclear substances and prescribed equipment in Canada remains safe.
Compliance verification
In 2017, CNSC staff conducted 944 inspections across the five sectors, including 160 security inspections to verify compliance with security expectations including incremental requirements outlined in REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources, for Category 1 and 2 sealed sources.
Overall, licensees showed satisfactory compliance ratings in all of the SCAs examined in this report. Where non-compliances were identified, CNSC staff ensured that licensees took appropriate corrective actions. Any non-compliances that had immediate risks to health, safety or security were addressed immediately by licensees. The majority of inspected licensees in 2017 were found to be compliant with the requirements in the SCAs covered in this report:
- In “management system”, 97% of the licensees inspected ensured that adequate processes and programs were in place to achieve their safety objectives.
- In “operating performance”, 85% of the licensees inspected made adequate provisions for the health, safety, security, and protection of the environment.
- In “radiation protection”, 85% of the licensees inspected had measures and programs in place to ensure that exposure to workers and the public to ionizing radiation was monitored, controlled and remained ALARA (as low as reasonably achievable).
- In “security”, 90% of the licensees inspected demonstrated that they have adequate provisions in place to prevent the loss, sabotage, illegal use, illegal possession or illegal removal of nuclear substances and prescribed equipment in their care and control.
- In “environmental protection”, 100% of the waste nuclear substance licensees inspected managed and monitored environmental emissions in a satisfactory manner.
As part of the phased implementation of REGDOC-2.12.3, security inspections were conducted for licensees in possession of high-risk sources in 2017; 73% of the inspections resulted in fully satisfactory or satisfactory ratings for the regulatory requirements imposed by REGDOC-2.12.3. Licensees have put in place measures to correct all non-compliances noted during these inspections. Details of the security inspections, such as non-compliances, are not provided in this report due to their sensitive nature.
Compliance enforcement
The CNSC uses a graded approach to enforcement to compel compliance and deter future non-compliances. When non-compliance (or continued non-compliance) has been identified, CNSC staff assess the significance of the non-compliance and determine the appropriate enforcement action, based on the CNSC’s graded approach to enforcement.
In 2017, the CNSC took 24 escalated compliance enforcement actions against licensees in the five sectors, including 18 orders and six administrative monetary penalties (AMPs). Most of the enforcement actions were taken against licensees in the industrial sector, consistent with trends from previous years. CNSC staff reviewed corrective measures implemented by all of the licensees to which orders were issued and found them to be satisfactory. Five of the six AMPs have been paid.
Effective doses to workers
Licensees are required to keep radiation doses to persons below CNSC regulatory limits and ALARA in accordance with the radiation protection programs established under the CNSC licences.
In 2017, doses were monitored for 53,350 workers in the five sectors covered in this report. Of those workers, 19,184 were designated as nuclear energy workers (NEWs). The remaining 34,166 were not designated as NEWs, and are referred to as non-NEWs in the report. Exposures to radiation continued to be very low for workers in 2017, consistent with previous reporting years.
One NEW in the medical sector received an extremity dose of 2,366 mSv (millisieverts) from handling a contaminated cart in a clinic. This dose was above the regulatory limit of 500 mSv. The incident was reported to the Commission in April 2017, and additional details can be found in section 5.7 of the report.
Reported events
CNSC staff assessed the 146 events reported by licensees covered in this report. Reported events have been ranked using the International Nuclear and Radiological Event Scale. Of these, 144 were ranked as level 0 (no safety significance), 1 was ranked as level 1 (anomaly) and 1 was ranked as level 2 (incident).
For all of the events reported, licensees implemented appropriate response measures to mitigate the impacts of the events and to limit radiation exposure to workers and the public. CNSC staff reviewed the measures and found them to be satisfactory.
Conclusion
Based on the CNSC’s comprehensive regulatory oversight of the industry, CNSC staff conclude that the use of nuclear substances in Canada is safe. Licensees corrected identified non-compliances to the satisfaction of CNSC staff; adequate provisions are in place for the protection of the health, safety and security of persons and the environment from the use of nuclear substances.
1 Background
1.1 Background
The Canadian Nuclear Safety Commission (CNSC) regulates the use of nuclear energy and materials to protect health, safety, security and the environment; to implement Canada’s international commitments on the peaceful use of nuclear energy; and to disseminate objective scientific, technical and regulatory information to the public. Persons licensed by the CNSC are responsible for operating their facilities and managing their activities safely, and are required to implement programs that make adequate provisions for protecting health, safety, security and the environment. The CNSC is responsible for setting the requirements and verifying compliance against those requirements.
Each year, CNSC staff assess the overall safety performance of the use of nuclear substances in Canada. Staff consider industry performance as a whole, as well as the performance of each sector (i.e., medical, industrial, academic and research, commercial, and waste nuclear substance) separately. This assessment is summarized in this document.
For a comprehensive overview of the CNSC and its activities, consult the CNSC’s annual report, Safety First.
Regulatory oversight
The CNSC regulates the nuclear industry in Canada through a comprehensive program of licensing, certification, compliance verification and enforcement. For each of the nuclear sectors described in this report, CNSC staff evaluate safety performance through assessments, inspections, reviews, and evaluations of licensee programs and processes.
These regulatory programs cover various types of activities across all provinces and territories, as shown in figure 1. Licensees include hospitals; universities and research institutions; and a wide variety of industrial manufacturing and production facilities including those that store, produce or service nuclear substances and devices, and operate and service prescribed equipment; and installations that store and process low-level waste from nuclear facilities and activities.
The safe use of nuclear substances in Canada is demonstrated through licensees’ compliance with the Nuclear Safety and Control Act (NSCA) and its associated regulations, as well as specific conditions set out in CNSC licences. The NSCA, its regulations and the licences require that licensees implement and maintain appropriate programs to ensure the safety and security of nuclear-related activities, minimize doses to workers and the public, protect the environment and minimize consequences of events.
Figure 1: Distribution of licensees in the provinces and territories of Canada
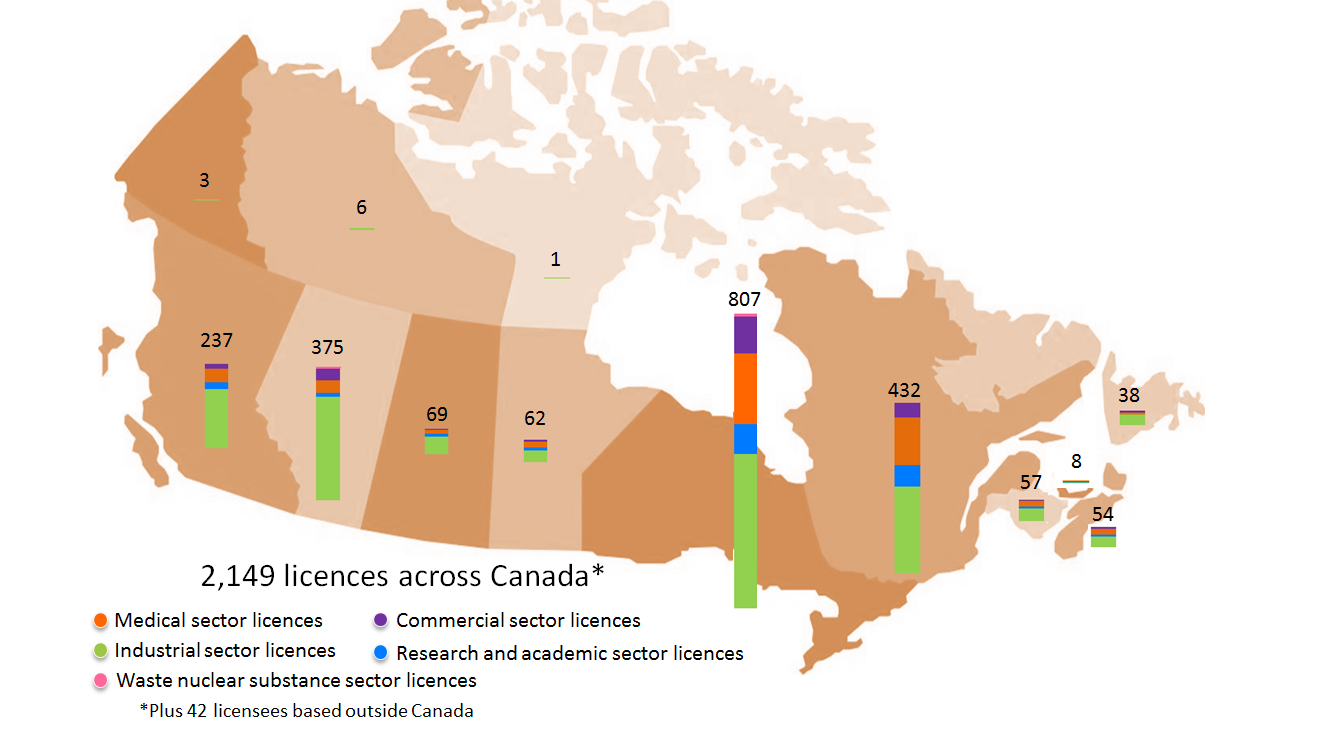
Description
Some licensees that hold CNSC licences to service radiation devices or prescribed equipment are based outside Canada and come to Canada to perform maintenance or servicing work on equipment owned by other licensees. When they work in Canada, these licensees are subject to the same level of regulatory oversight from the CNSC as those whose operations are based in Canada.
2 Report overview
This regulatory oversight report focuses on the results of compliance verification and enforcement activities in 2017 for licensees authorized for activities involving nuclear substances or prescribed equipment. For the purposes of reporting, licensees are grouped in five sectors:
- medical
- industrial
- academic and research
- commercial
- waste nuclear substance
Each sector’s performance is outlined in an individual section in this report.
The waste nuclear substance licensees included in this report handle low-level waste from research laboratories, as well as slightly contaminated metals, laundry and equipment from other types of nuclear facilities.
There are three parts to this report:
- regulatory process and developments
- overall safety performance assessment
- sector-specific safety performance assessments
2.1 Safety and control area framework
To ensure comprehensive regulatory oversight and reporting of licensed activities, CNSC staff have developed a set of safety and control areas (SCAs). SCAs have been in use for a number of years, and represent a well-established set of technical areas that have proven effective in evaluating licensee safety performance of regulated facilities and activities under the CNSC’s purview. The CNSC has defined 14 SCAs:
- management system
- human performance management
- operating performance
- safety analysis
- physical design
- fitness for service
- radiation protection
- conventional health and safety
- environmental protection
- emergency management and fire protection
- waste management
- security
- safeguards and non-proliferation
- packaging and transport
2.2 Safety performance measures
During licensing and compliance activities, CNSC staff review the licensee’s (or applicant’s) performance within each relevant SCA by reviewing licensee documents and conducting inspections. The broad nature of the different activities conducted by licensees covered by this report means that not all SCAs apply to all activities or all licensees.
For the purpose of this report, the performance in a subset of the SCAs is reviewed. The following four SCAs are the most relevant indicators of safety performance for licensees in the sectors covered in this report: management system, operating performance, radiation protection and security. In addition, ratings for the environmental protection SCA are included for the waste nuclear substance sector. Environmental protection SCA ratings are not reported for the other sectors because the majority of nuclear substance and radiation device licensees are authorized to possess and use sealed sources and radiation devices, which have no impact on the environment (as per REGDOC-2.9.1, Environmental Protection: Environmental Principles, Assessments and Protection Measures, version 1.1). Sealed sources are designed to international standards and must meet stringent design requirements. The CNSC certifies all radiation devices to ensure that they are safe for use and meet CNSC requirements, which includes an assessment of their design. In addition, the CNSC regulations require regular leak testing of sealed sources, thereby ensuring that the environment is protected.
The unsealed nuclear substances, used by a small group of CNSC licensees, are short-lived radionuclides. Their use is confined to controlled rooms or laboratories designed in accordance with regulatory requirements, including associated work practices, that prevent the release or uncontrolled release of nuclear substances to the environment. These measures form a part of the licensing basis for the CNSC licence issued. CNSC staff verify compliance with these measures through compliance activities.
For waste nuclear substance licensees that may have interactions with the environment, releases to the environment are monitored and reported to the CNSC. Environmental releases are minimized through the use of emissions control technologies such as HEPA (high-efficiency particulate air) filters and waste water collection tanks, which is consistent with the principles outlined in REGDOC‑2.9.1. Emissions from waste nuclear substance licensees have historically been below levels that would pose a risk to the public or the environment. CNSC staff are satisfied that there are adequate measures in place to protect the public and the environment.
Performance in the packaging and transport SCA is not explicitly covered in the report. However, events related to packaging and transport are included in the report, and provide an indication of their impacts to health and safety of persons and to the environment. CNSC staff review all events reported and ensure licensees implement adequate corrective measures. Compliance ratings – also referred to as inspection ratings – reflect overall licensee performance for the SCAs covered in this report. The nature, type and safety significance of events reported by licensees, as well as the type of enforcement actions taken by the CNSC in 2017, are provided as supplementary indicators of safety performance. Data from 2013 to 2017 are included in figures for each of these safety indicators in order to identify five-year trends. Each performance measure is described below.
Although not incorporated into this report, all relevant SCAs are assessed during compliance inspections and reviews of licensees’ documents, and a compliance rating similar to those found in this report is assigned for each SCA. All required corrective actions arising from below-satisfactory performance are tracked and followed up by CNSC staff to ensure that all non-compliances are addressed to the satisfaction of the CNSC.
2.2.1 Doses to workers
Each licensee is required to implement a radiation protection program that ensures that the radiation doses to workers are well below regulatory limits and kept ALARA (as low as reasonably achievable), with social and economic factors taken into account. Thus, ascertainment of the magnitude of doses received by workers is an integral part of a licensee’s radiation protection program.
This report references two groups of workers that perform the types of activities referenced in a CNSC licence: those designated as nuclear energy workers (NEWs) and those not designated as NEWs (non-NEWs). The term “NEW” means a person who is required, in the course of his or her business or occupation in connection with a nuclear substance or nuclear facility, to perform duties in circumstances that may result in receiving a dose of radiation greater than 1 millisievert (mSv) per year. A worker not designated as a NEW means a person is unlikely to receive a dose greater than 1 mSv per year while performing duties in connection with a nuclear substance or nuclear facility. This report provides dose information for all workers, while primarily focusing on those designated as NEWs.
The CNSC’s regulatory effective dose limits for NEWs are set at 50 mSv in any one-year dosimetry period and a total of 100 mSv over a five-year dosimetry period. The one-year dosimetry period covers January 1 to December 31 of every year. The current five-year dosimetry period started on January 1, 2016 and will end on December 31, 2020. For all persons not designated as NEWs, and for all members of the general public, the effective dose limit is 1 mSv per calendar year.
Additionally, the CNSC’s Radiation Protection Regulations set out equivalent dose limits for the lens of the eye, the skin, and the hands and feet, for NEWs and all other persons.
Appendix A provides more information on occupational exposure, ascertaining worker doses and measures to be taken by licensees when a dose limit is exceeded.
2.2.2 Management system
The management system SCA covers the framework that establishes the processes, programs and resources required to ensure that a licensee achieves its safety objectives, continuously monitors its performance against those objectives, and fosters a healthy safety culture.
2.2.3 Operating performance
Operating performance refers to the licensee’s ability to perform licensed activities in accordance with pertinent operational and safety requirements defined in the NSCA, its associated regulations and licence conditions. Licensees are expected to demonstrate that they comply with operational and safety requirements by providing workers with appropriate procedures for the safe use of nuclear substances and prescribed equipment, by ensuring that workers follow procedures and by maintaining records that demonstrate compliance.
2.2.4 Radiation protection
Radiation protection programs are required for every licensee to ensure that contamination levels and radiation doses received by workers are monitored, controlled and maintained below regulatory dose limits, and kept ALARA, with social and economic factors taken into account. Licensees can meet these objectives by monitoring worker doses; posting radiation warning signs; planning appropriately for radiological emergencies; managing oversight of operational activities; and instituting effective workplace practices that emphasize the use of time, distance and shielding to minimize exposure to radiation, and emphasize the use of appropriate protective equipment.
2.2.5 Environmental protection
The environmental protection SCA covers the measures licensees have in place to manage and monitor any environmental emissions. The scale and complexity of the environmental protection program is commensurate with the environmental risk associated with the licensed activity.
2.2.6 Security
The security SCA covers the physical security measures, practices and programs that licensees are required to have in place to prevent the loss, illegal use, illegal possession or illegal removal of nuclear substances during their entire lifecycle, including while they are in storage or during transport. The extent of the security measures required depends upon the types of nuclear substances used and activities performed by each licensee.
The safety and security of sealed sources is increased through effective control and tracking. CNSC compliance inspections include requirements to verify sealed source tracking information.
To ensure proper regulatory oversight of the requirements related to the first phase of the implementation of REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources, CNSC staff conduct security inspections for those in possession of Category 1 and 2Footnote 1 sealed sources. Non-compliance details for these inspections are not included in this report due to their sensitive nature.
2.2.7 Enforcement actions
The CNSC may take a variety of enforcement actions to ensure that licensees correct non-compliances in an effective and timely manner. The type of enforcement action taken is commensurate with the risk the non-compliance presents to the environment, the health and safety of workers and the public, and to national security. This report provides detailed information on the following types of enforcement actions taken by the CNSC: orders, administrative monetary penalties (AMPs), decertification of certified exposure device operators and decertification of radiation safety officers at Class II nuclear facilities. Appendix B provides a list of all orders and AMPs issued to nuclear substance and radiation device licensees by the CNSC in 2017.
No exposure device operators or Class II radiation safety officers were decertified in 2017.
2.2.8 Reported events
Under the NSCA and its associated regulations, licensees are required to immediately report to the CNSC events related to their licensed activities that are of regulatory interest. Within 21 days of becoming aware of the incident, licensees are required to submit a written report to the CNSC on the event. The written report must include an analysis of the cause and circumstances of the event, as well as any measures taken, or proposed to be taken, by the licensee to prevent recurrence. Together, the initial and final reports allow the CNSC to verify whether the licensee has taken appropriate measures to mitigate the event and implemented adequate corrective actions to prevent recurrence.
The CNSC uses the International Nuclear and Radiological Event Scale (INES) tool to categorize events in the sectors covered by this report. Additional information on the INES classification can be found on the CNSC website.
2.3 Data collection
Compliance ratings, non-compliance data, and CNSC enforcement actions were obtained from the CNSC’s compliance verification and enforcement program in 2017.
Annual compliance reports submitted by licensees in calendar year 2017 provided the data on doses incurred by all persons engaged in licensed activities in the five sectors covered in this report.
2.4 Glossary
For definitions of terms used in this document, see REGDOC‑3.6, Glossary of CNSC Terminology, which includes terms and definitions used in the Nuclear Safety and Control Act and the regulations made under it, and in CNSC regulatory documents and other publications.
2.5 Changes to the 2017 regulatory oversight report
CNSC staff introduced the following additions to this year’s report:
-
Waste nuclear substance licensees have been included as a fifth sector.
- These are licensees authorized by the designated officer to manage, handle, store and process low-level radioactive waste.
- Previously, these licensees were reported as part of the Regulatory Oversight Report for Waste Management, Storage and Processing in Canada: 2015.
- The relative risk of subsectors highlighted in the report has been included.
3 Regulatory program for the use of nuclear substances
The possession, use, transfer, import, export, abandonment and storage of nuclear substances must be licensed by the Canadian Nuclear Safety Commission (CNSC) when the amount of nuclear substance involved is greater than its exemption quantity (see Schedule 1 of the Nuclear Substances and Radiation Devices Regulations). Facilities where certain types of Class II prescribed equipment is to be installed must also be licensed by the CNSC prior to their construction, operation or decommissioning. A licence is also required to service radiation devices or Class II prescribed equipment.
All licensees that operate Class II nuclear facilities or that service Class II prescribed equipment must have a certified radiation safety officer (RSO) and a qualified temporary replacement. The RSO is responsible for implementing the radiation protection program, and ensuring that licensed activities are conducted safely and all regulatory requirements are met.
All radiation devices and most Class II prescribed equipment, as well as certain types of transport packages, must be certified by the CNSC before they can be used in Canada.
The CNSC’s compliance and verification program measures licensee compliance with CNSC regulatory requirements. Regular inspections and desktop evaluations verify that licensees comply with the Nuclear Safety and Control Act (NSCA) and its associated regulations, as well as any conditions included in their licences.
To determine appropriate levels of regulatory monitoring and control, CNSC staff establish compliance verification plans for each nuclear sector that are based on risk-informed regulatory oversight of each sector’s activities. Modifications to the compliance plans are made on an ongoing basis in response to events and changes in licensees’ performance.
For the activities covered in this report, the CNSC’s risk-informed regulatory program is applied in the following way:
- Each licensed activity is assigned a weighting factor – a coefficient that represents the activity’s relative significance with respect to risk.
- Factors considered in weighting include the form of the nuclear substances (e.g., sealed source, unsealed source or radiation device), the location where the material is being used (e.g., a work site or a controlled facility), and the compliance history of licensees conducting licensed activities.
- Generally, licensees are inspected on a one- to five-year cycle, based on their risk ranking.
The risk-informed regulatory program provides:
- a risk ranking that recognizes the potential safety impact of the licensed activity
- effective and informed allocation of regulatory oversight effort according to the risk ranking by licensed activity and by licensee performance history
- effective, transparent, consistent and comprehensive regulatory oversight
3.1 CNSC regulatory effort
The CNSC’s risk-informed regulatory program applies resources and regulatory oversight commensurate with the risk associated with the regulated activity. Regulatory effort related to licensing, certification and compliance verification is derived from this program. A total of 944 inspections were completed in 2017, compared to 1,452 inspections in 2016. The decrease in the number of inspections is related to several factors, including:
- transition to radiation protection program reviews through an increase in Type I inspections, which provide a broader assessment of licensee program performance, although with substantially higher effort per inspection ‒ this is consistent with the radiation protection program oversight strategy presented to the Commission in 2016
- increased focus on full inspection coverage of licensee locations in order to verify licensee compliance at remote locations, with increased travel time associated with each inspection
- completion of security inspection campaign for the first phase of implementation of REGDOC-2.12.3 for high-risk sealed sources
- increased mobility of inspectors in 2017, leading to the development of a staffing strategy to hire and train new inspectors in order to manage long-term impacts on the compliance program
The CNSC takes a risk-informed approach to compliance verification, whereby the planning and execution of compliance activities are commensurate with the risk of the licensed activity. High-risk licensees continue to be prioritized for inspections, and all high-risk inspections were completed per the inspection plan.
Inclusion of an additional sector, the waste nuclear substance licensees, had a minimal impact on the total number of inspections, as only four inspections were conducted in this sector in 2017.
Type I inspections of Class II licensees are conducted as per the risk-informed regulatory program.
As shown in table 1, CNSC staff direct effort for regulating the use of nuclear substances and prescribed equipment in 2017 amounted to close to 13,059 person-days, or the annual equivalent of approximately 58 full-time staff.
| Activity | Person-days |
|---|---|
| Licensing | 4,602 |
| Certification | 1,629 |
| Compliance verification | 7,280 |
3.2 Licensing
To obtain a licence, an applicant must submit an application to the CNSC. The CNSC will issue a licence only when the applicant:
- is deemed qualified to carry on the activity that the licence will authorize
- has demonstrated that they will protect the health and safety of persons and the environment
- has demonstrated that they will maintain national security
- has confirmed that they will adhere to international obligations to which Canada has agreed
CNSC staff perform a risk-informed technical assessment of applications submitted to the CNSC.
The CNSC has produced a series of licence application guides to ensure that its expectations for applicants are clear, and to facilitate applicants’ interactions with the regulator. These guides are reviewed on a five-year cycle as part of the regulatory framework review to ensure that they continue to reflect modern regulatory expectations and provide useful guidance to the regulated community. This practice, in turn, facilitates CNSC licensing reviews and minimizes regulatory burden. Application forms and guides for nuclear substances and radiation devices as well as for Class II facilities and prescribed equipment can be found on the CNSC website.
When applying for licence renewals, existing licensees are subject to the same scrutiny as new applicants. The CNSC decision to renew a licence is based on the application information submitted, such as ensuring that there are no outstanding cost recovery fees and the financial guarantee is up to date, and there is a satisfactory compliance history. This includes a review of compliance information such as inspection results, reported incidents and events, and annual compliance reports. A peer review by another CNSC staff member is conducted following the initial review of all licence applications.
If the application satisfies the above requirements, the Commission, or a designated officer authorized by the Commission, may issue a licence authorizing the licensee to conduct the activities requested in the application. The licence includes provisions that define and limit the scope of the authorized activities, as well as specific conditions that must be fulfilled by the licensee when conducting those activities.
The number of licences by sector is shown in table 2. The number of licences issued for activities covered by this report continues to decrease. There are fewer licensees in each of the medical, industrial, and academic and research sectors. The CNSC’s policy to consolidate licences where appropriate is one driver of this reduction, particularly in the medical and academic and research sectors. In the industrial sector, however, the decrease in the number of licences is driven to a greater extent by the economic conditions and business decisions of the licensees, including larger companies acquiring smaller ones and some licensees moving to non-nuclear technologies.
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Medical | 552 | 536 | 494 | 470 | 457 |
| Industrial | 1,440 | 1,398 | 1,349 | 1,308 | 1,287 |
| Academic and research | 232 | 229 | 207 | 208 | 195 |
| Commercial | 256 | 248 | 245 | 247 | 246 |
| Waste nuclear substance | 8 | 8 | 6 | 7 | 6 |
| Total | 2,488 | 2,419 | 2,301 | 2,231 | 2,191 |
3.3 Certification of prescribed equipment
An application for certification must be submitted to the CNSC before the prescribed equipment can be used in Canada. CNSC staff who conduct the technical evaluations of applications for certification are accredited as professional engineers. Upon receipt of an application, CNSC staff conduct a thorough technical review of the information contained in the submission to determine if:
- the radiation device, Class II prescribed equipment or transport package meets all CNSC regulatory requirements and is safe to use
- adequate measures are in place in respect of its use to protect the environment, national security, and the health and safety of persons
A CNSC quality assurance program in the form of a peer review by another CNSC staff member is in place for the review of applications for certification of prescribed equipment.
If satisfied that the design meets the above requirements, the Commission, or a designated officer authorized by the Commission, may issue a certificate for the prescribed equipment based on the recommendation of the CNSC staff members who conducted the technical evaluations.
If the design does not comply with the above requirements or if a certified model is found to be unsafe, the designated officer will contact the applicant and all affected parties, such as users in the case of a certified model, to inform them of the decision to either not certify the new model or to decertify a currently certified model. In these cases, the CNSC will provide the applicant and affected parties an opportunity to be heard in accordance with the process specified in the regulations.
Regulatory documents REGDOC-1.5.1, Application Guide: Certification of Radiation Devices or Class II Prescribed Equipment and RD/GD-352, Design, Testing and Performance of Exposure Devices outline CNSC expectations for the certification of radiation devices and Class II prescribed equipment, while RD/GD- 364, Joint Canada – United States Guide for Approval of Type B(U) and Fissile Material Transportation Packages (currently under review), outlines CNSC expectations for the certification of transport packages.
3.4 Certification of exposure device operators
Licensees are required under the Nuclear Substances and Radiation Devices Regulations to permit only CNSC-certified personnel and supervised trainees to use exposure devices containing nuclear substances. In 2017, the CNSC certified 87 new exposure device operators (EDOs) and renewed the certifications of 302 others. EDOs must renew their certification every five years to ensure they maintain the knowledge and skills required to operate an exposure device safely.
The CNSC EDO certification program is designed to ensure the continued competency of the operator, and maintain the safety and security of persons and devices when working with exposure devices. Certified individuals must demonstrate the ability to:
- handle, transport, store and operate exposure devices and any accessories to the devices safely and securely
- properly utilize radiation detection and monitoring equipment
- understand the obligation to comply with all relevant regulatory requirements
Figure 2: CNSC inspector observes a certified exposure device operator preparing for industrial radiography on a pipeline (Source: CNSC)

Regulatory document REGDOC-2.2.3, Personnel Certification: Exposure Device Operators, and CSA Group document CSA PCP-09, Certified Exposure Device Operator Personnel Certification Guide (under review), outline the CNSC’s requirements and guidance for certification as an EDO and for renewal of an EDO certification. In 2017, CNSC staff updated the EDO certification application forms and the CNSC Web page on EDO certification. The EDO application forms were redesigned in order to standardize the information submitted in EDO applications to ensure that the CNSC receives all of the information necessary to process the applications. The new forms have reduced the need for CNSC staff to request applicants to provide additional information to support their EDO applications, and have reduced the time needed to process EDO certification applications.
The CNSC may take regulatory action if an EDO is found to be operating contrary to safety protocols and conditions, or if an EDO is causing undue risk to the public or the environment. No EDOs were decertified in 2017.
3.5 Certification of Class II radiation safety officers
All licensees that operate Class II nuclear facilities or that service Class II prescribed equipment must have a certified radiation safety officer (RSO) and a qualified temporary replacement. The RSO ensures that licensed activities are conducted safely and all regulatory expectations are met.
There are two components to the RSO certification process:
- an assessment of the candidate’s capability to perform the duties of the position, based on the submitted application
- an assessment of the candidate’s knowledge of the licensed activities, based on an examination
RSO candidates must possess certain qualifications before they can be considered for certification. For most Class II licensed activities, candidates must have at least a bachelor’s degree in engineering or science from a recognized university.Footnote 2 Alternative education qualifications may be reviewed on a case-by-case basis.
If the candidate is able to clearly demonstrate their knowledge as it relates to the RSO position within their organization, the Commission or a designated officer authorized by the Commission may certify the candidate in the position of RSO.
The process for certification of Class II RSOs, along with guidance for applicants, is outlined in REGDOC-2.2.3, Personnel Certification: Radiation Safety Officers.
In 2017, the CNSC certified 28 applicants as Class II RSOs. No Class II RSOs were decertified in 2017. The CNSC has certified 240 Class II RSOs since 2010.
3.6 RSO appointment for nuclear substances and radiation devices licences
There are approximately 1,660 RSOs appointed for nuclear substances and radiation devices licences. The designation of an RSO for nuclear substances and radiation devices licences is the responsibility of the applicant authority, the person accountable for the management and control of the licensed activity. The RSO is the person the CNSC will contact about radiation safety and compliance matters. The appointment of these RSOs does not involve a certification process.
The CNSC requires that the RSO’s qualifications be included in a licence application, and will determine if the RSO has sufficient knowledge and expertise with regard to the applicant’s proposed activities. The RSO may be a consultant hired by the applicant to carry out this role, provided that the consultant is clearly designated by the applicant authority to do so. Such information must be communicated to the CNSC as part of the licence application process. Site RSOs may be utilized where a licensee has multiple locations of licensed activity.
Unless otherwise noted by the applicant authority, the RSO will be considered to have the authority to act for the applicant and will have signing authority for all matters encompassed by the CNSC licence.
For new applicants of licences for high-risk activities (for example, industrial radiography, well logging), CNSC staff perform additional verifications as part of the licensing process. They meet with the designated RSO and the applicant authority during a pre-licensing visit to verify the RSO’s knowledge of the licensee’s proposed radiation protection program and confirm that the applicant understands their obligations as a licensee. CNSC staff plan visits and prepare the interview following review of the application and the applicant’s radiation protection program. During the visit, CNSC staff ensure that the licensee understands the radiation protection program that has been committed to and review the location of the proposed licensed activities. At the same time, CNSC staff ensure that candidates have strong radiation safety knowledge as well as advanced training in operational and emergency procedures. If it is deemed that the appointed RSO does not have adequate knowledge, the licensing decision will be withheld pending the appointment of a suitable RSO. In 2017, CNSC staff performed nine pre-licensing visits for prospective nuclear substance and radiation device licensees. In two cases, CNSC staff found deficiencies in either the RSO’s knowledge of the radiation protection program or the radiation program itself. In these cases, the licence was not issued until the concerns were addressed to the satisfaction of CNSC staff.
3.7 RSO appointment for waste nuclear substance licences
Each waste nuclear substance licensee designates an individual who is responsible for implementing the licensee’s radiation protection program: the radiation protection program authority. The responsibilities of the position are the same, regardless of whether the job title is “radiation safety officer” or something else.
3.8 Licensing and certification decisions
CNSC designated officers made a total of 2,571 licensing and certification decisions related to activities covered in this report in 2017. The majority of these were licensing decisions, as shown in table 3.
There was an increase in the number of certification activities in 2017 due to the expiry and recertification of a higher-than-average number of device certificates, a trend that began in 2016. In 2018, the number of device certifications is expected to drop back to levels seen prior to 2015.
After peaking at 455 in 2016, the number of decisions related to the certification of exposure device operators (EDOs) returned to what is expected to be normal levels of approximately 400 per year. The increased number of EDO certification renewals in 2016 was attributed to the CNSC’s gradual implementation of a new expectation that came into effect in 2015 for EDOs to renew their certification every five years as per CSA Group document CSA PCP-09, Certified Exposure Device Operator Personnel Certification Guide. In 2018, the number of EDO certifications is expected to be similar to the 2017 levels, as the EDO certification and certification renewal programs as per CSA PCP-09 have matured and started to stabilize.
| Type of decision | Number of decisions |
|---|---|
| Licensing (issuance of new licences, licence renewals, licence amendments, licence revocations and licence transfers) | 1,972 |
| Certification of prescribed equipment (radiation devices, Class II prescribed equipment and transport packages) | 182 |
| Certification of EDOs (issuance of new certification and renewal of certification) | 389 |
| Certification of Class II RSOs | 28 |
| Total | 2,571 |
3.9 Compliance verification and enforcement
The CNSC verifies compliance by conducting site inspections and reviewing licensee documentation and operational activities. Licensees are required to report routine performance data through annual compliance reports and the occurrence of specific types of events. In addition, the CNSC conducts investigations of unplanned events, public complaints or accidents involving nuclear substances.
The CNSC uses a graded approach to enforcement to encourage compliance and deter future non-compliances. When a non-compliance (or a continued non-compliance) has been identified, CNSC staff assess its risk and safety significance to determine appropriate enforcement action. The chosen enforcement action is commensurate with the risk that the non-compliance presents to the environment, the health and safety of workers and members of the public, and to national security. Enforcement actions vary with non-compliance severity, and can include orders and administrative monetary penalties (AMPs). Each is a discrete and independent response to a non-compliance.
Escalated enforcement actions were taken 24 times in 2017 against licensees in the sectors covered in this report. The majority were in response to inspection findings.
In 2017, CNSC staff conducted 944 inspections to verify compliance with CNSC regulatory requirements, including 160 security inspections to verify compliance against the requirements of REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources, for Category 1 and 2 sealed sources.
3.10 Stakeholder engagement
Clarity of requirements is one of the CNSC’s corporate priorities. Stakeholder engagement and outreach are two tools the CNSC uses to meet this priority. Outreach and engagement lead to an increased awareness and better understanding of the regulatory process and requirements, which, in turn, lead to increased workplace safety. CNSC staff take every opportunity to perform outreach, including while on inspection. Furthermore, to improve the public’s level of understanding of proposed or licensed nuclear facilities and activities, some Class II licensees are required to develop and implement a public information program that includes a disclosure protocol.
CNSC outreach sessions held throughout Canada in 2017 gave licensees and others the opportunity to interact with the regulator outside the scope of an inspection or licensing activities. This past year, digital technologies such as WebEx were used to host virtual outreach sessions and working group meetings. Some of the key sessions are described below. In addition to outreach sessions, CNSC staff delivered presentations at various conferences to share information on developing regulatory topics.
Figure 3: CNSC information booth at the Family Medicine Forum in Montréal, QC
3.10.1 Outreach to licensees
Outreach sessions
Since 2009, the CNSC has offered an outreach program for licensees that use nuclear substances and prescribed equipment. The presentations made by CNSC staff and discussions associated with outreach are meant to inform licensees and other persons regulated by the CNSC about recent and upcoming regulatory changes, and provide education regarding the CNSC’s expectations for licensing and compliance requirements. In 2017, outreach sessions were held in Winnipeg, Manitoba and Vancouver, British Columbia.
Newsletters
In 2009, the CNSC introduced the DNSR newsletter as an outreach vehicle for disseminating regulatory and safety information to licensees that use nuclear substances and prescribed equipment in Canada. The newsletter articles address various regulatory compliance issues and support the regulator’s commitment to keep both licensees and the public informed. Regular editions of the newsletter provide valuable information to licensees in all sectors; special editions focus on either a specific subsector or an area of regulatory interest.
All newsletters are posted on the CNSC website and are sent to recipients on the CNSC subscription list.
In 2017, two editions of the DNSR newsletter were published: a spring edition in June and a fall edition in December. Topics included information for licensees on how and when to report an event to the CNSC; operating experience and lessons learned from the nuclear medicine and portable gauge industries; information about implementation of REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources, for users of Category 3, 4 and 5 sealed sources; a summary of regulatory actions; a summary of events reported to the International Atomic Energy Agency (IAEA) by other countries; and a summary of events reported to the CNSC.
Safety posters
In 2017, CNSC staff published updated safety posters on laboratory classifications, spill procedures, responding to accidents involving portable gauges, and the proper use and care of personal dosimeters.
3.10.2 Outreach to the medical sector
Canadian Radiation Protection Association working group
A working group was established between the CNSC and the Canadian Radiation Protection Association (CRPA) in 2014. In 2017, this working group continued its efforts to promote strong radiation safety cultures within licensed activities. Topics of discussion at meetings this past year included proposals for licence consolidation of nuclear substance and radiation device licences, the evaluation the CNSC is conducting on success factors for a radiation protection program, and upcoming changes to regulatory documents.
Canadian Radiation Protection Association meeting
For almost three decades, CNSC staff have delivered regulatory-focused presentations and participated in regulatory workshops at the CRPA’s annual conferences. At the 2017 annual meeting, CNSC staff delivered presentations on identifying success factors of the RSO, the benefits of internal inspections to the radiation protection program, upcoming licensing process reforms and care of decedents. Furthermore, CNSC staff participated in a town hall session to answer questions from conference attendees.
Canadian Organization of Medical Physicists
The Canadian Organization of Medical Physicists (COMP) represents medical physicists working in radiotherapy facilities in the medical sector. Many certified radiation safety officers at Class II nuclear facilities are members of COMP.
CNSC staff attended the 2017 COMP Annual Scientific Meeting in Ottawa, Ontario. CNSC staff participated in a panel discussion on the importance of the RSO and factors influencing success in that role.
CNSC-Class II/CRPA/COMP working group
The CNSC-Class II/CRPA/COMP (C3) working group was established in late 2015 with the mission of providing a forum for communication and information sharing among stakeholders in the regulated Class II community. The group met twice in 2017. Topics discussed included methods for communicating with applicant authorities, the workload of RSOs, the importance of internal audits, and ways to communicate with members of the CRPA and COMP.
Other outreach activities
CNSC staff met with the Cancer Care Ontario RSO Community of Practice to discuss the proposed changes to the licence application guide for Class II licences.
CNSC staff delivered a presentation to the nuclear medicine community at The Ottawa Hospital about the CNSC’s mandate and activities, and specifically about the Directorate of Nuclear Substances Regulation. CNSC staff described how the CNSC conducts inspections of nuclear medicine licensees and provided case studies of challenges associated with nuclear medicine.
On three occasions, CNSC staff hosted webinars for medical licensees that focused on the full implementation of REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources.
The CNSC hosted an information booth at the Family Medicine Forum held in Montréal, Quebec. CNSC staff provided interested attendees with both information on the CNSC’s role in regulating activities in the medical sector, and resources on ionizing radiation and radiation doses.
CNSC staff held a webinar that, while not specific to the medical field, informed funeral industry professionals regarding the proper care of decedents who received radiation therapies or nuclear medicine treatments prior to their death.
3.10.3 Outreach to the industrial sector
Industrial radiography working group
In 2009, a CNSC/industrial radiography working group was established to foster improved communications between the CNSC and the industry. The working group meets twice a year to discuss best practices and safety performance, and provides a forum in which stakeholders can stay informed of new developments from both technical and regulatory perspectives. At the 2017 meetings, the group discussed items of interest to the industrial radiography community, including ways to communicate with and educate industrial radiography clients.
Industrial radiography annual meeting
The CNSC holds two separate annual meetings with the radiography industry. In 2017, the meetings were held in Nisku, Alberta, and Ottawa, Ontario. CNSC staff use these meetings to address recent and upcoming regulatory developments and discuss other areas of regulatory focus. During the 2017 meetings, the CNSC gave presentations on its expectations for radiation protection programs, the process for the certification of exposure device operators (EDOs) and an overview of compliance for the previous year. Representatives of industry delivered presentations to their peers on the experience of conducting internal audits and on the EDO practical examination.
Certified Exposure Device Operator Scheme Committee
In 2016, the CSA Group formed a scheme committee consisting of CNSC staff, Natural Resources Canada staff and representatives from the industrial radiography community to discuss potential updates to CSA Group document CSA PCP-09, Certified Exposure Device Operator Personnel Certification Guide. This committee met in 2016 to discuss committee members’ experience with CSA PCP-09 and to provide suggestions for improvements to the document. CNSC staff are currently using the suggestions from this meeting as a basis to update CSA PCP-09. CNSC staff plan to issue an update to CSA PCP-09 to the CSA Scheme Committee for review in 2018.
In 2017, the CSA Group hosted a working group meeting composed of Scheme Committee members and subject matter experts to review and update the bank of questions and answers for the EDO written examination.
3.11 International commitments
CNSC staff continue to collaborate with international counterparts to support IAEA activities by participating in working groups and technical meetings, contributing to the technical guidance documents and advisory missions, and delivering training programs in other member states. CNSC staff participated in developing a peer review strategy for Africa, providing training in Lithuania, and supporting the development of a number of IAEA safety standards and guides.
In fall 2017, in partnership with the IAEA, the CNSC hosted a three-week training course for Caribbean countries on establishing and strengthening sustainable regulatory infrastructures for the control of radiation sources. Representatives from nine Caribbean countries participated in classroom and field training delivered by CNSC staff. Participants received training on topics that ranged from the Code of Conduct on the Safety and Security of Radioactive Sources to the fine details of inspection and investigation of nuclear medicine sources and equipment, nuclear gauges, industrial radiography and safe transport.
The Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management (Joint Convention) was the first legal instrument to directly address these issues on a global scale and was opened for signature on September 29, 1997. Canada was one of the first countries (known as Contracting Parties) to ratify the Joint Convention, which came into force on June 18, 2001.
The Joint Convention applies to spent fuel and radioactive waste resulting from civilian nuclear reactors and applications. The Joint Convention also applies to planned and controlled releases into the environment of liquid or gaseous radioactive materials from regulated nuclear facilities.
The obligations of the Contracting Parties with respect to the safety of spent fuel and radioactive waste management are based to a large extent on the principles contained in the IAEA Safety Fundamentals document, The Principles of Radioactive Waste Management, published in 1995. They include, in particular, the obligation to establish and maintain a legislative and regulatory framework to govern the safety of spent fuel and radioactive waste management, and the obligation to ensure that individuals, society and the environment are adequately protected against radiological and other hazards, by appropriate siting, design and construction of facilities, and by making provisions for ensuring the safety of facilities both during their operation and after their closure. The Joint Convention imposes obligations on Contracting Parties in relation to the transboundary movement of spent fuel and radioactive waste based on the concepts contained in the IAEA Code of Practice on the International Transboundary Movement of Radioactive Waste. Also, Contracting Parties have the obligation to take appropriate steps to ensure that disused sealed sources are managed safely.
The CNSC coordinates and submits the national reports on behalf of Canada. These reports represent a collective work and involve the cooperation of various federal departments, as well as input from licensees and industry organizations. Canada’s national reports are published together with responses to questions received from other Contracting Parties. All Canadian national reports can be found on the CNSC’s website. Canada’s sixth national report was submitted in October 2017 and was presented at the Sixth Review Meeting in May 2018. CNSC staff will present the outcome of the Joint Convention to the Commission in fall 2018.
4 Regulatory developments
This section provides details of the regulatory developments of 2017 relating to regulatory programs for licensees covered in this report.
4.1 Licence application guide
In May 2017, REGDOC-1.6.1, Licence Application Guide: Nuclear Substances and Radiation Devices , Version 2 was published. The document provides applicants with guidance on completing and submitting an application for a nuclear substance and radiation devices licence. This revision includes changes to areas of the document that CNSC staff and applicants identified as needing clarification. The changes include:
- the addition of graphic warnings for information that should not be submitted by email (dose information, security information, social insurance numbers)
- an update to the financial guarantee information
- improvements to the forms associated with the licence application guide in order to reduce repetition of information required by the applicant and to automatically add the licence number to all pages of the forms
4.2 Certification of prescribed equipment
REGDOC-2.5.7, Design, Testing and Performance of Exposure Devices, was published in August 2017. The document provides guidance on the design, testing and performance of exposure devices in order to apply for certification of the device.
The regulatory document supersedes RD/GD-352, Design, Testing and Performance of Exposure Devices. The changes to the document were administrative in nature.
4.3 Exposure device operators
In 2017, there were two significant regulatory developments affecting exposure device operators (EDOs). They are described briefly below.
REGDOC-2.2.3, Personnel Certification: Exposure Device Operators, was published in March 2017. The document provides a reference to CSA PCP‑09, which documents the CNSC’s requirements and guidance for certification as an EDO. This regulatory document supersedes G-229, Certification of Exposure Device Operators.
With the first full cycle of renewals of exposure device operators completed in 2016, the Canadian Nuclear Safety Commission (CNSC) clarified its position with all industrial radiography licensees that workers are only considered qualified if they have a valid EDO certification card, that is, an EDO certification card issued on or after February 1, 2013 that has not expired. As a result, any EDO whose certification card either does not have an expiry date or has expired will not be qualified to work as a certified EDO as of January 1, 2017, and may be subject to enforcement actions.
4.4 Radiation safety officers
In 2017, CNSC staff started reviewing the oversight process of RSOs who are appointed (i.e., those who do not need to sit and pass an examination) in order to identify factors that may lead to greater success in that position. This process will be undertaken using internationally accepted methods and will be designed with the assistance of experts from within and outside the CNSC. It is anticipated that results from the review will be included in a regulatory document that will provide those performing the role of RSO with greater guidance on the CNSC’s expectations for individuals occupying the position of RSO.
4.5 Regulatory focus in 2018
The CNSC’s focus in 2018 continues to be on effective regulatory oversight and continuous improvement. Activities that will be undertaken in 2018 include:
-
verifying the implementation of the requirements in REGDOC‑2.12.3,
Security of Nuclear Substances: Sealed Sources, which came into force on May 31, 2018 for Category 3, 4
and 5 sealed sources (REGDOC‑2.12.3, has been in force for Category 1 and 2 sealed sources since 2015)
- proactively engaging with licensees that will be affected to prepare them for any changes to requirements that will take effect on that date
- continuing the strategy for enhancing the oversight of radiation safety officers and radiation protection programs for nuclear substance and radiation device licensees that was presented to the Commission in October 2017
- rolling out an information program targeting portable gauge users, including an updated user booklet and a safety video that have been developed to address the trends of decreasing compliance and the relatively high number of events, relative to other subsectors
- continuing to monitor the regulatory framework and regulatory practices to ensure that they remain appropriate, agile and sufficient to effectively regulate new technologies, new applications of existing technologies and new types of prescribed equipment
-
developing the first revision of CSA Group document CSA PCP-09,
Certified Exposure Device Operator Personnel Certification Guide
- The revised document will be distributed to the industry stakeholders for review and comments prior to its implementation
-
finalizing the following regulatory documents, which were posted for public comment in 2017 and are expected to
be published in 2018:
- REGDOC-1.4.1, Licence Application Guide: Class II Nuclear Facilities and Prescribed Equipment
- REGDOC-1.5.1, Licence Application Guide: Certification of Radiation Devices or Class II Prescribed Equipment
- REGDOC-2.1.2, Safety Culture
- REGDOC-2.5.5, Design of Radiography Installations
- REGDOC-2.7.3, Radiation Protection Guidelines for Safe Handling of Decedents
5 Safety performance – all sectors combined
This section provides an overview of the overall performance of the industry sectors covered in this report.
5.1 Overall safety assessment
Canadian Nuclear Safety Commission (CNSC) staff conducted 944 inspections across all sectors in 2017 to verify compliance with CNSC regulatory requirements, including 160 security inspections to verify enhanced security requirements applicable to Category 1 and 2 sealed sources. All sectors continued to demonstrate adequate performance within all safety and control areas (SCAs). The majority of inspected licensees in 2017 were found to be compliant in the five SCAs covered in this report:
- In management system, 97% of the licensees inspected ensured that adequate processes and programs were in place to achieve their safety objectives.
- In operating performance, 85% of the licensees inspected made adequate provisions for the health, safety, security, and protection of the environment.
- In radiation protection, 85% of the licensees inspected had measures and programs in place to ensure that exposure to workers and the public to ionizing radiation was monitored, controlled and remained ALARA (as low as reasonably achievable).
- In security, 90% of the licensees inspected demonstrated that they have adequate provisions in place to prevent the loss, sabotage, illegal use, illegal possession or illegal removal of sealed sources and prescribed equipment in their care and control.
- In environmental protection, 100% of waste nuclear substance licensees continue to manage and monitor environmental releases resulting from licenced activities. Releases were kept well below regulatory limits and there were no unplanned releases to the environment as a result of licensed activities (section 10).
REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources, added new requirements for security of sealed sources. Phase I of the implementation plan for the regulatory document focused on Category 1 and 2 sealed sources, and came into force for those licensees in May 2015. Since then, security inspections of licensees in possession of high-risk sealed sources have verified compliance with the requirements in REGDOC‑2.12.3. In 2017, 73% of inspected licensees (117 of 160 inspections) were found to be compliant with the requirements of REGDOC‑2.12.3. This is a slight decrease compared to the level of compliance in 2016. The majority of non-compliances for security requirements were related to deficiencies in the site security plan, non-effective physical barriers for storage locations and inadequate measures of access control. Licensees have put in place measures to correct all non-compliances identified during these inspections. To date, all licensees with Category 1 and 2 sealed sources have been inspected, although not all work locations have been inspected.
Doses for 53,351 workers were reported to the CNSC in the five sectors covered in this report. Of those workers, 19,185 were designated as nuclear energy workers (NEWs), while 34,166 were not.
There was one instance of overexposure in 2017. A nuclear medicine technologist received a dose to the skin of the hand that was above regulatory limits. Additional information is provided in the sections on the medical sector (section 6.3.1).
For 2017, CNSC staff assessed all 146 events reported by the licensees covered in this report. Reported events have been ranked using the International Nuclear and Radiological Event Scale (INES). Of these, 144 were ranked as level 0 (no safety significance), 1 was ranked as level 1 (anomaly) and 1 was ranked as level 2 (incident).
For all of the events reported, licensees implemented appropriate response measures to mitigate the impacts of the events and to limit radiation exposure to workers and the public. CNSC staff reviewed the measures put in place by licensees and found them to be satisfactory.
In 2017, 12 inspections of a total of 10 licensees received a rating of unacceptable in at least one SCA. Seven of the licensees were portable gauge licensees, considered a medium-risk activity. One licensee was in the industrial radiography sector, considered a high-risk activity, one was involved with fixed gauges (a medium-risk activity) and one did servicing (a medium-risk activity). The CNSC took escalated enforcement actions, issuing an order, an administrative monetary penalty (AMP) or both, in all but one case. Additional details about enforcement actions can be found in section 5.6. All orders are closed. All but one AMP have been paid.
5.1.1 Licensees with compliance ratings below expectations
The CNSC takes a licensee’s compliance history into account when determining the inspection schedule. When a licensee repeatedly performs below expectations, the CNSC may increase the regulatory oversight of the licensee. This could include additional reactive inspections to monitor ongoing compliance or increased inspection frequency. The CNSC reviewed the past performance of licensees that had poor ratings in at least one SCA in 2017; 21% of these licensees (11 out of 53) were rated below expectations or unacceptable in the same SCA in their previous inspection. All were medium-risk licensees. In all cases, CNSC inspectors tracked the items of non-compliance until they were addressed by the licensee in a manner that was satisfactory to CNSC staff. The poor performance of these licensees was considered when determining the inspection plan for the fiscal year 2018/19.
5.2 Management system
The management system SCA covers the framework that establishes the processes and programs required to ensure that an organization achieves its safety objectives, continuously monitors its performance against those objectives and fosters a healthy safety culture.
All sectors demonstrated satisfactory performance within the management system SCA, with 97% of inspected licensees (840 of 865 inspections) found to be in compliance with regulatory requirements (see figure 4). This is consistent with previous years. A breakdown of the inspection ratings by sector for 2017 is shown in figure 5.
Two inspections resulted in unacceptable ratings for the management system SCA for one licensee from the commercial sector and one licensee from the industrial sector. A CNSC inspector issued an order to the licensee from the industrial sector.
The majority of non-compliances in this SCA were the result of licensees failing to submit an annual compliance report as required; failing to keep required records at all work locations, including any temporary work locations; and conducting activities for which they were not licensed (generally, these items were administrative in nature, such as using nuclear substances not listed on their licences or possessing radiation device models that did not appear on their licences). CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 4: Inspection ratings for management system, 2015–17
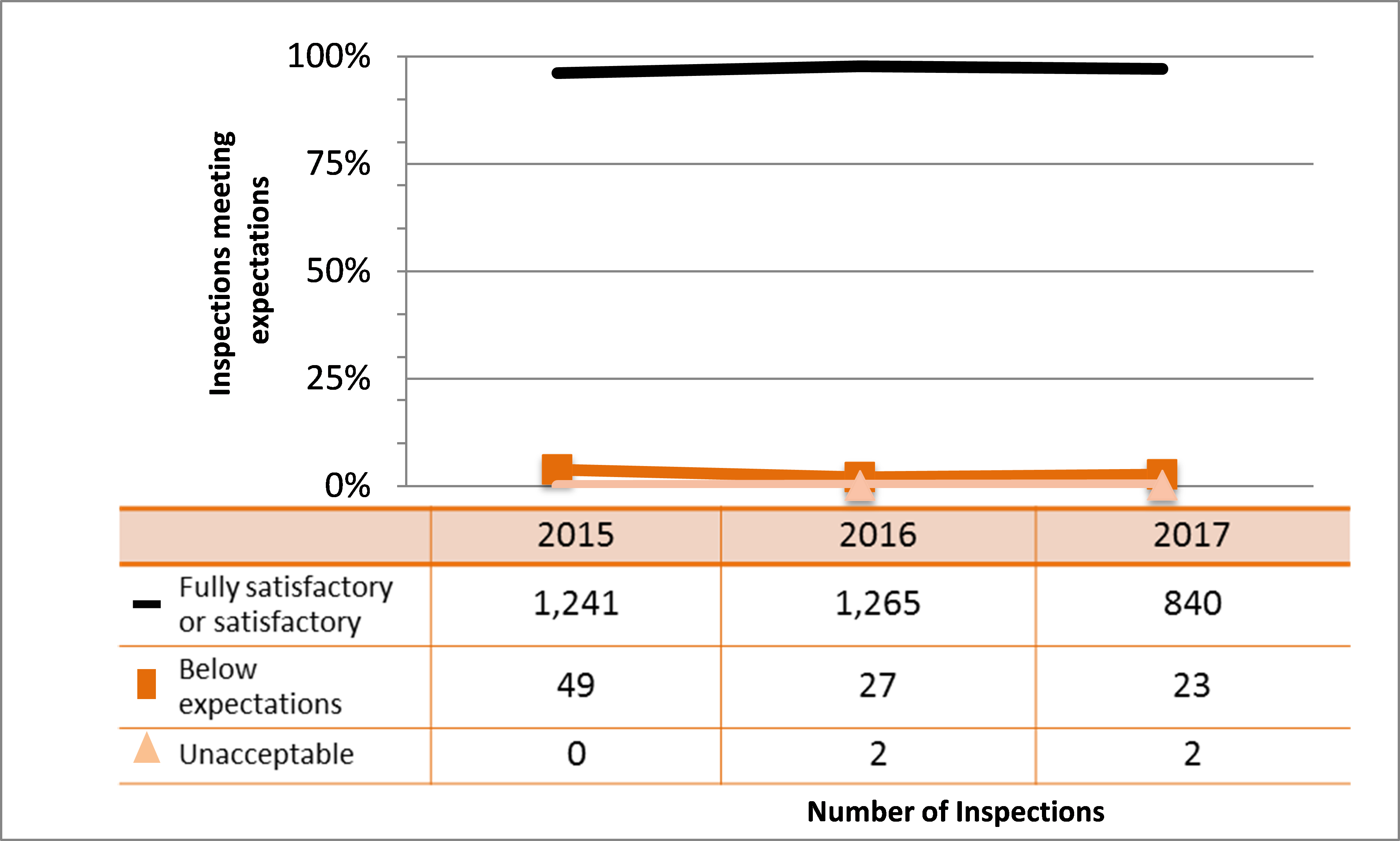
Description
| Ratings | 2015 | 2016 | 2017 |
|---|---|---|---|
| Fully satisfactory or satisfactory | 1,241 | 1,265 | 840 |
| Below expectations | 49 | 27 | 23 |
| Unacceptable | 0 | 2 | 2 |
Figure 5: Sector-to-sector comparison of inspection ratings meeting or exceeding expectations for management system, 2015–17
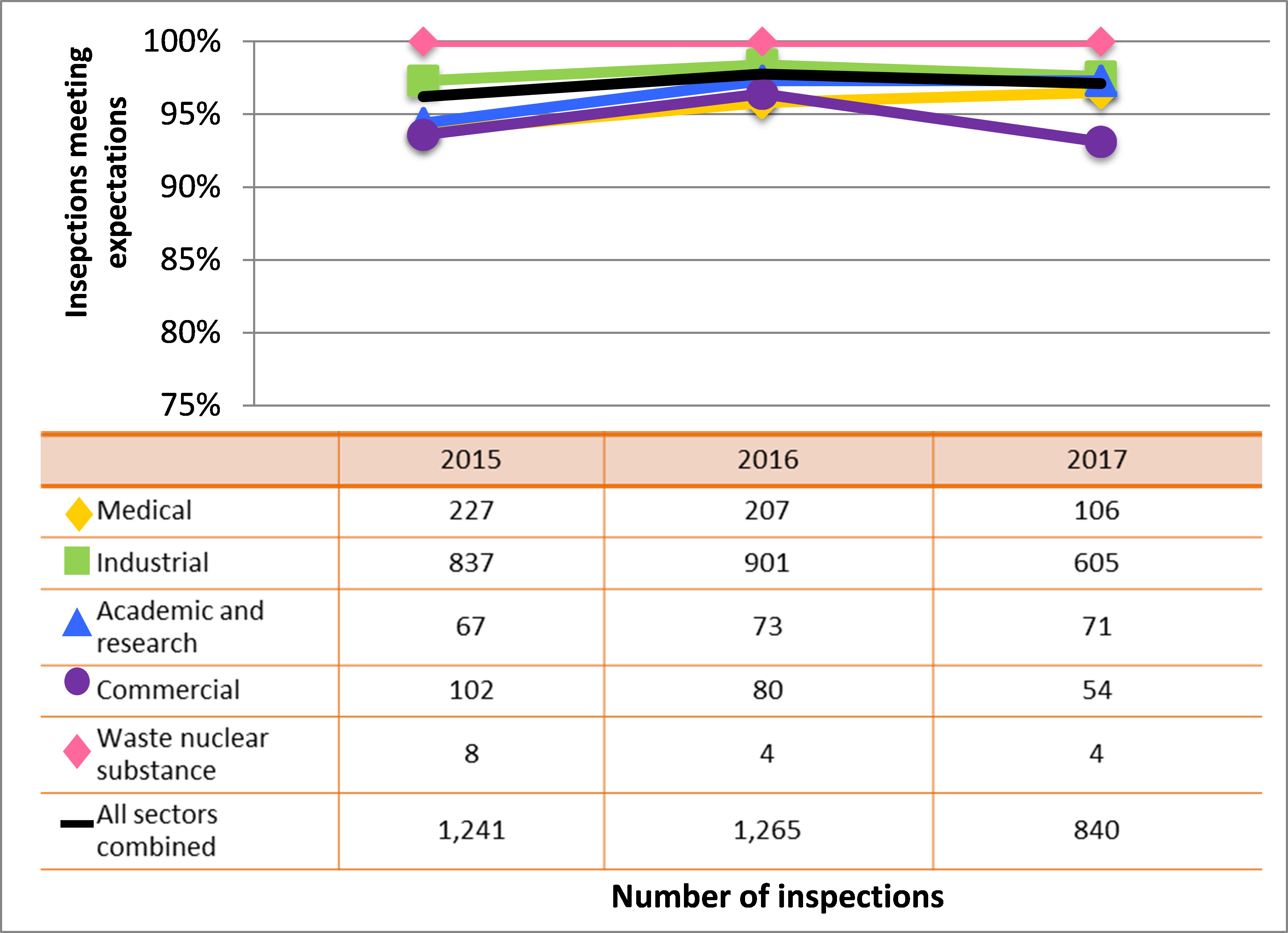
Description
| Sector | 2015 | 2016 | 2017 |
|---|---|---|---|
| Medical | 227 | 207 | 106 |
| Industrial | 837 | 901 | 605 |
| Academic and research | 67 | 73 | 71 |
| Commercial | 102 | 80 | 54 |
| Waste nuclear substance | 8 | 4 | 4 |
| All sectors combined | 1,241 | 1,265 | 840 |
5.3 Operating performance
Operating performance refers to the licensee’s ability to perform licensed activities in accordance with pertinent operational and safety requirements defined in the Nuclear Safety and Control Act (NSCA), its associated regulations and licence conditions. Licensees are expected to demonstrate that they comply with operational and safety requirements by providing workers with appropriate procedures for the safe use of nuclear substances and prescribed equipment, by ensuring that workers follow procedures, and by maintaining records that demonstrate compliance.
All sectors continued to demonstrate adequate performance within the operating performance SCA in 2017, with 85% of inspected licensees (747 out of 883 inspections) found to be in compliance with regulatory requirements. This continues a gradual downward trend in performance for this SCA. Specific projects are in place to address the decreasing performance in the medical and industrial sectors, are described in sections 4.4 and 7.3.3, respectively. These projects, the RSO and Radiation Protection Program Evaluation project, and initiatives targeting portable gauge users, aim to improve the performance across all SCAs.
Inspection ratings for all sectors combined are shown in figure 6, and a sector-to-sector comparison appears in figure 7. Performance of both the medical and industrial sectors dropped again in 2017, reducing overall performance in this SCA. The academic and research sector showed improved performance again in 2017. Its performance in this SCA has rebounded above where it was in 2013 when it started to decline.
Eight inspections resulted in a rating of unacceptable for the operating performance SCA in 2017. In all cases, inspectors issued orders to the licensees to stop the unsafe work practices and ensure corrective actions were taken immediately.
The most common non-compliances in this SCA included failure to comply with regulatory requirements related to workers’ responsibilities to follow licensee procedures and use equipment provided by the licensee, failure to follow the procedures in the documents appended to the licence, failure to keep training records for employees, and failure to conduct leak testing of sealed sources at the required frequency. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 6: Inspection ratings for operating performance, 2013‒17
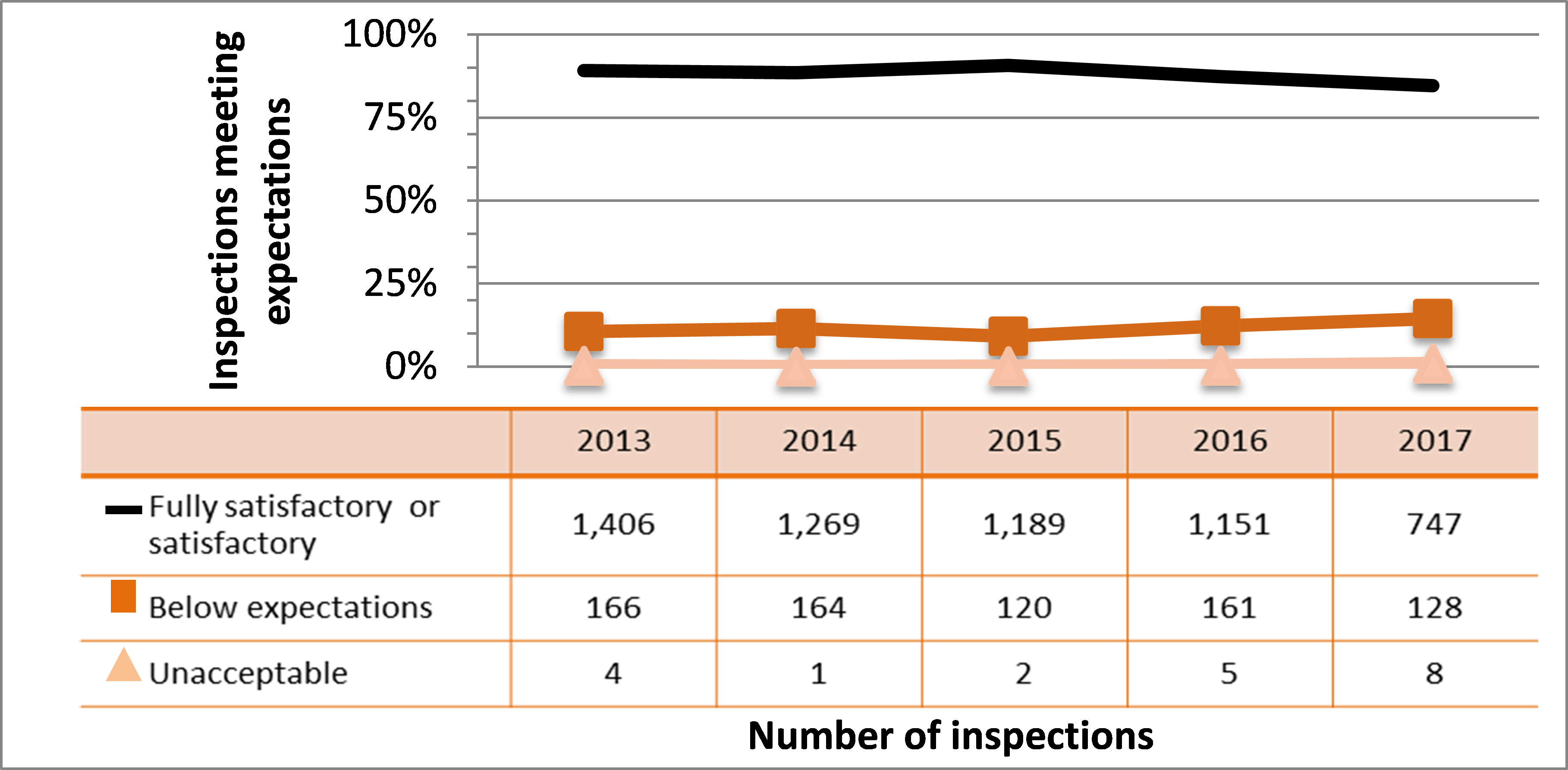
Description
| Rating | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory | 1,406 | 1,269 | 1,189 | 1,151 | 747 |
| Below expectations | 166 | 164 | 120 | 161 | 128 |
| Unacceptable | 4 | 1 | 2 | 5 | 8 |
Figure 7: Sector-to-sector comparison of inspection ratings meeting or exceeding expectations for operating performance, 2013–17
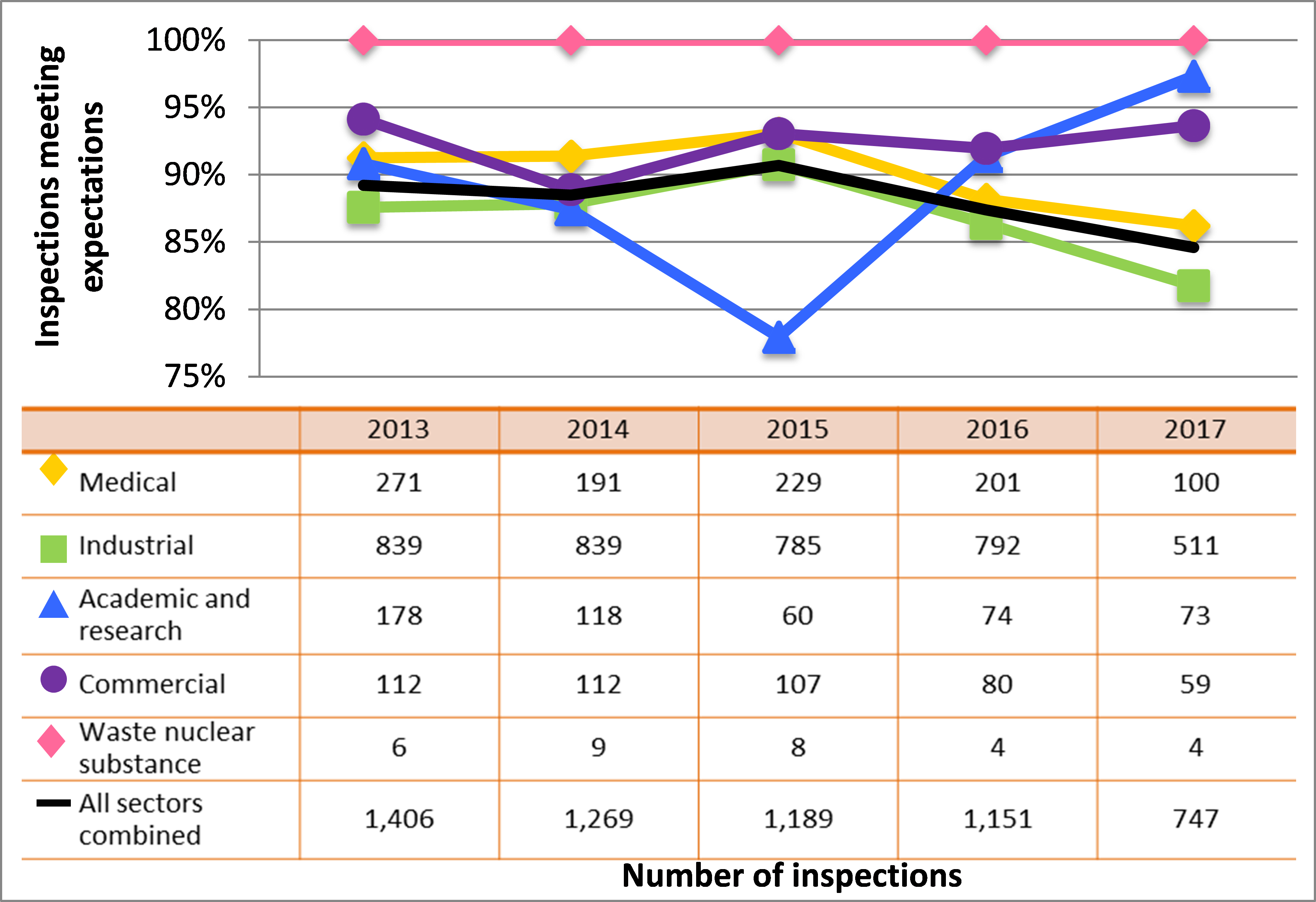
Description
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Medical | 271 | 191 | 229 | 201 | 100 |
| Industrial | 839 | 839 | 785 | 792 | 511 |
| Academic and research | 178 | 118 | 60 | 74 | 73 |
| Commercial | 112 | 112 | 107 | 80 | 59 |
| Waste nuclear substance | 6 | 9 | 8 | 4 | 4 |
| All sectors combined | 1,406 | 1,269 | 1.189 | 1,151 | 747 |
5.4 Radiation protection
Figure 8: Checking for contamination during an inspection (Source: CNSC)

Radiation protection programs are required for every licensee to ensure that contamination levels and radiation doses received by workers are monitored, controlled and maintained below regulatory dose limits, and kept at levels that are ALARA, social and economic factors being taken into account. Licensees are expected to monitor worker doses; post radiation warning signs; plan appropriately for radiological emergencies; manage oversight of operational activities; and institute effective workplace practices that emphasize the use of time, distance and shielding to minimize exposure to radiation, and emphasize the use of appropriate protective equipment.
All sectors demonstrated adequate performance within this SCA, with 85% of inspected licensees (744 of 876 inspections) found to be compliant with regulatory requirements. However, this represents a lower level of compliance than has been observed since 2013. (See figure 9). The drop in performance in this SCA is driven by the medical and industrial sectors. Further details about measures being taken to improve the performance of these sectors can be found in sections 6.3 and 7.3, respectively.
In 2017, three inspections resulted in unacceptable ratings for radiation protection. Of these inspections, one also had an unacceptable rating for the operating performance SCA, and another one had an unacceptable rating for the management system SCA. In all three cases, the CNSC inspectors issued an order to immediately stop the unsafe work practices and to implement corrective measures.
Inspection ratings for all sectors combined are shown in a sector-to-sector comparison presented in figure 10.
The most common reasons for non-compliance were failing to implement radiation programs that keep doses to workers and the public ALARA, failing to post radiation warning signs as required, failing to limit access to storage areas to authorized workers and failing to keep the dose rate outside storage areas below the regulatory limit. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 9: Inspection ratings for radiation protection, 2013–17
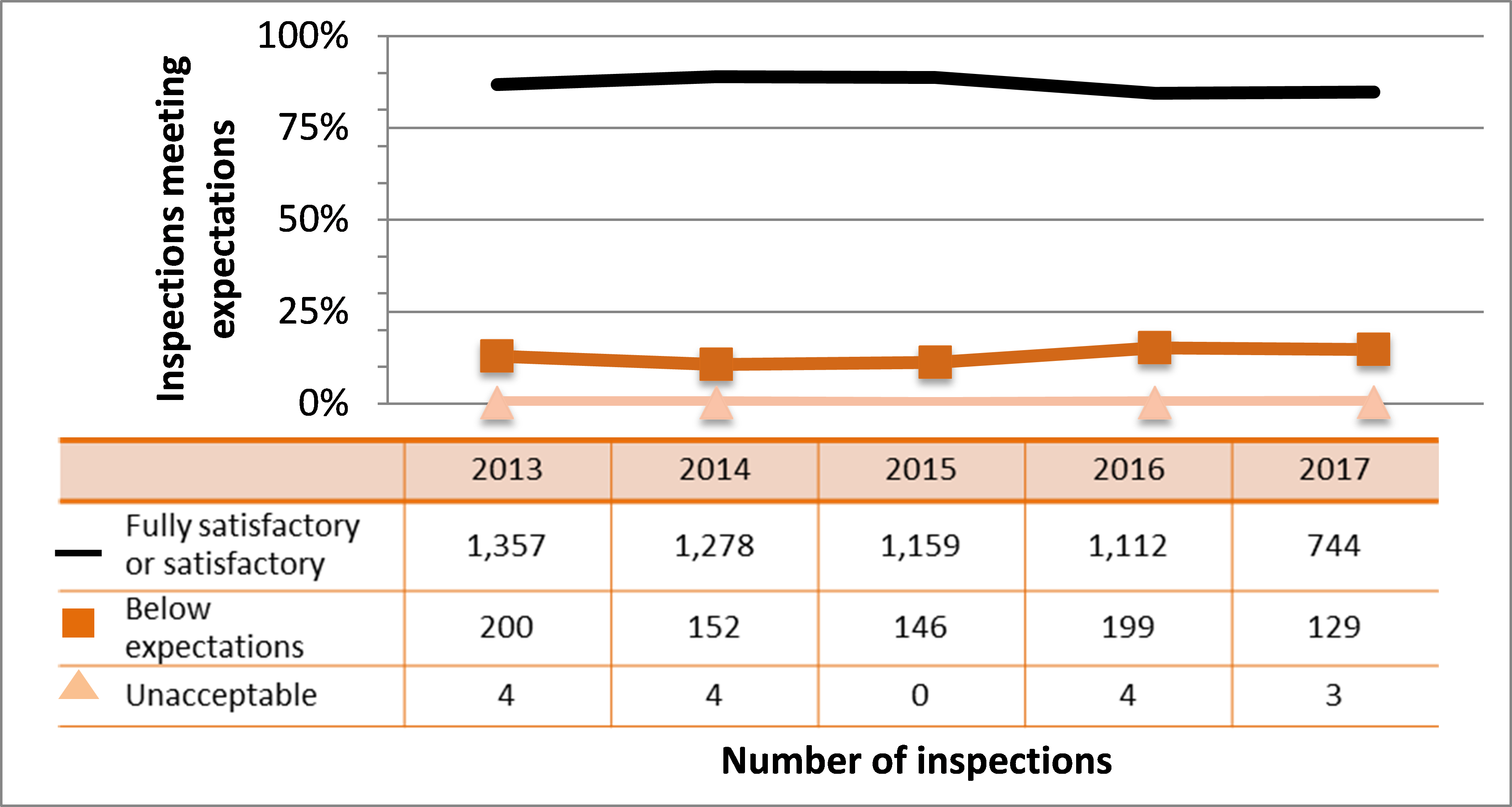
Description
| Rating | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 1,357 | 1,278 | 1,159 | 1,112 | 744 |
| Below expectations | 200 | 152 | 146 | 199 | 129 |
| Unacceptable | 4 | 4 | 0 | 4 | 3 |
Figure 10: Sector-to-sector comparison of inspection ratings meeting or exceeding expectations for radiation protection, 2013–17
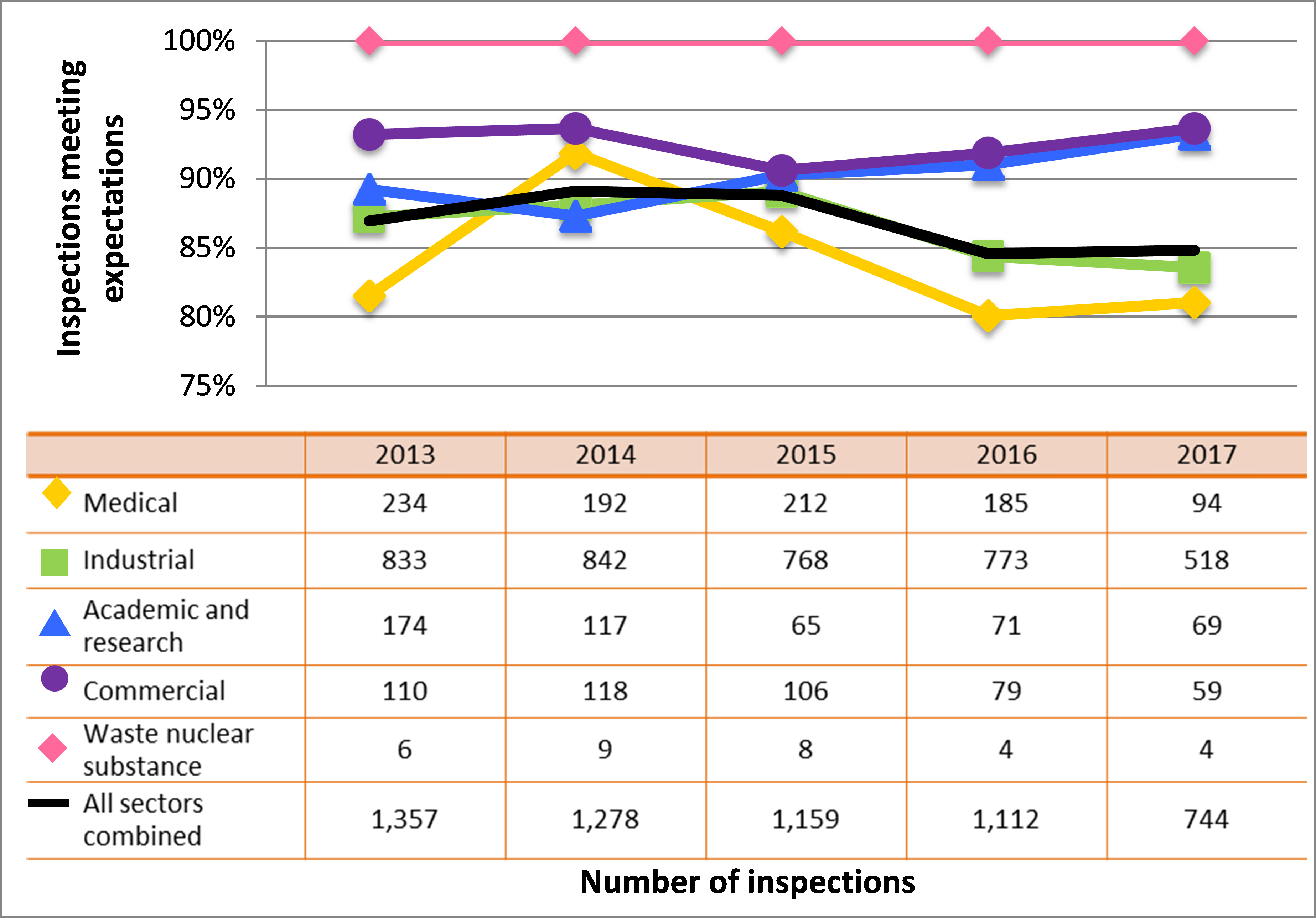
Description
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Medical | 234 | 192 | 212 | 185 | 94 |
| Industrial | 833 | 842 | 768 | 773 | 518 |
| Academic and research | 174 | 117 | 65 | 71 | 69 |
| Commercial | 110 | 118 | 106 | 79 | 59 |
| Waste nuclear substance | 6 | 9 | 8 | 4 | 4 |
| All sectors combined | 1,357 | 1,278 | 1,159 | 1,112 | 744 |
5.5 Security
Licensees are required to have in place physical security measures, practices and programs to prevent the loss, illegal use, illegal possession or illegal removal of nuclear substances during their entire lifecycle, including while they are in storage or during transport, as per the NSCA. The extent of the security measures required depends upon the types of nuclear substances used and activities performed by each licensee.
Overall, all sectors showed satisfactory ratings for the security SCA in 2017: 90% of inspected licensees (762 of 848 inspections) were compliant with regulatory requirements. This is lower than previous years. The decline can be attributed to lower rates of compliance in the medical, industrial and commercial sectors.
Nine inspections received unacceptable ratings in the security SCA. Eight of these inspections also received unacceptable ratings in the operating performance SCA. All licensees that received unacceptable grades for the security SCA were subject to enforcement actions.
Licensees with high-risk sealed sources are subject to the requirements described in REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources. After May 31, 2018, the document applies to licensees holding all categories of sealed sources. In 2017, CNSC inspectors conducted security inspections to verify compliance against the requirement applicable to Category 1 and 2 sealed sources; 73% (117 of 160 inspections) of the inspections resulted in fully satisfactory or satisfactory ratings with regulatory requirements for high-risk sealed sources. Generally, the licensees have in place basic physical security measures. Non-compliances were for requirements introduced in REGDOC-2.12.3, including deficiency with the site security plan, insufficient physical barriers at storage locations and inadequate access control measures. CNSC staff continue to work with licensees impacted by REGDOC-2.12.3 to clarify the regulatory expectations. These efforts include webinars, articles in the DNSR newsletter, and direct communication with licensees.
CNSC staff reviewed 128 site security plans for licensees covered by this report. In cases in which staff determined the plans were not acceptable, they worked with the licensees to address deficiencies. Site security plans were not accepted until they met the requirements of REGDOC-2.12.3.
Licensees addressed and corrected to the satisfaction of the CNSC all non-compliances identified during inspections. Figure 11 summarizes the performance of all sectors combined for this SCA for 2014 to 2017, while figure 12 provides a sector-to-sector comparison for those three years.
Figure 11: Inspection ratings for security, 2014–17
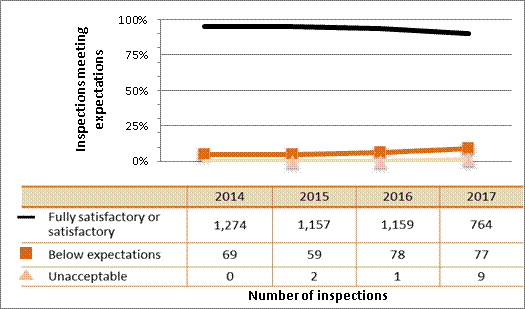
Description
| Rating | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| Fully satisfactory or satisfactory | 1,274 | 1,157 | 1,159 | 764 |
| Below expectations | 69 | 59 | 78 | 77 |
| Unacceptable | 0 | 2 | 1 | 9 |
Figure 12: Sector-to-sector comparison of inspection ratings meeting or exceeding expectations for security, 2014–17
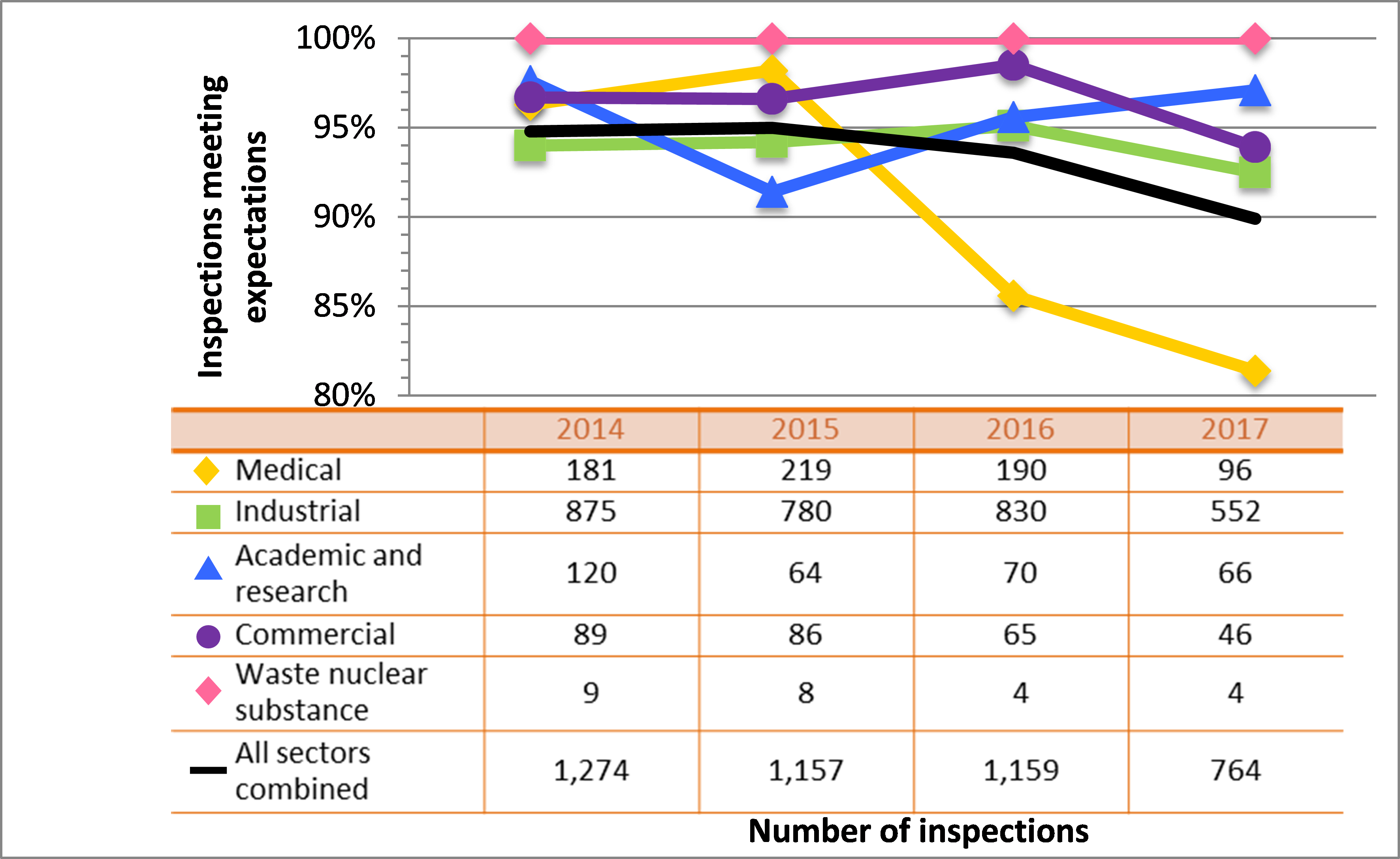
Description
| Sector | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| Medical | 181 | 219 | 190 | 96 |
| Industrial | 875 | 780 | 830 | 552 |
| Academic and research | 120 | 64 | 70 | 66 |
| Commercial | 89 | 86 | 65 | 46 |
| Waste nuclear substance | 9 | 8 | 4 | 4 |
| All sectors combined | 1,264 | 1,157 | 1,159 | 764 |
Compliance with the mandatory tracking of high-risk sealed sources was satisfactory in 2017. Of 133 inspected licensees, 130 (98%) were found to be compliant with this requirement. This is a higher level of compliance than was observed in 2016. CNSC staff ensured that all instances of non-compliance were adequately addressed by the licensees. The non-compliances for high-risk sealed sources were administrative in nature: not providing notifications within the required time frame and not keeping the records up to date. Further information on this topic is available in the National Sealed Source Registry and Sealed Source Tracking System Annual Report
5.6 Enforcement actions
In 2017, the CNSC took 24 escalated enforcement actions against licensees in the industrial and commercial sectors (figure 13). No enforcement actions were taken against licensees in the medical, academic and research, or waste nuclear substance sectors. CNSC staff issued orders directing licensees to take immediate corrective measures in 18 instances. Inspectors issued 17 orders; 1 was issued by a designated officer. All orders were issued to licensees in the industrial sector. In each case, the licensee complied with the order. Once the CNSC was satisfied that the licensee had addressed the terms and conditions of the order, it is considered closed. All orders issued in 2017 are closed.
CNSC designated officers issued administrative monetary penalties (AMPs) in six instances in 2017. Three of the AMPs were issued following or in conjunction with orders. Three AMPs were issued to individuals (two working in the industrial sector and one working in the commercial sector) and three to licensees, all in the industrial sector. Five of the six AMPs have been paid.
The sections describing the sectors provide a description of the enforcement actions issued in that sector. A summary of orders and AMPs issued by the CNSC in 2017 is provided in appendix B. Further information on regulatory actions taken by the CNSC, including escalated enforcement actions, is also available on the CNSC website.
Figure 13: Sector-to-sector comparison of CNSC enforcement actions, 2013–2017
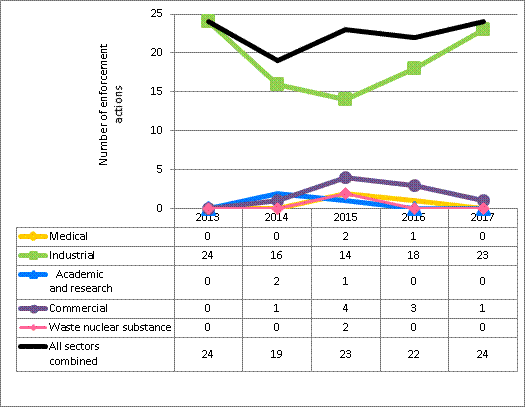
Description
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Medical | 0 | 0 | 2 | 1 | 0 |
| Industrial | 24 | 16 | 14 | 18 | 23 |
| Academic and research | 0 | 2 | 1 | 0 | 0 |
| Commercial | 0 | 1 | 4 | 3 | 1 |
| Waste nuclear substance | 0 | 0 | 2 | 0 | 0 |
| All sectors combined | 24 | 19 | 21 | 22 | 24 |
5.7 Reported events
Licensees are required to have programs in place for the management of unplanned events and accidents. The events that warrant mandatory reporting and the content of the reports are set out in the NSCA, its regulations and the licence conditions. CNSC staff review, assess and track all events reported by licensees.
Since 2014, reported events have been ranked using the (International Nuclear and Radiological Event Scale (INES)), a tool for communicating the safety significance of nuclear and radiological events to the public. This tool makes it possible to establish a proper perspective of an event’s safety significance. The scale has been used to classify events at nuclear power plants since 1990 and has been extended over the years to apply to all nuclear industry installations. By 2006, it had been adapted to all events associated with the transport, storage and use of radioactive sources and nuclear substances. Note that the scale is not a tool to compare safety performances among facilities or organizations, but to effectively communicate the safety significance of events.
In 2017, there were 146 events related to nuclear substances reported to the CNSC by licensees in the sectors covered in this report. Of these events, 144 were ranked as INES level 0 (no safety significance) and one was ranked as level 1 (anomaly) based on the quantity of nuclear substances involved and the type of event reported: a stolen portable gauge. The remaining event was ranked level 2. In this incident, a NEW received an extremity dose of 2,366 mSv to the skin of the hand, which is above the regulatory limit of 500 mSv. This was reported to the Commission in April 2017.
A breakdown of reported events by type is shown in figure 14 and a complete list of all reported events in 2017 is provided in appendix C.
For all of the events reported, the licensees implemented adequate response measures to mitigate the impacts of the events and to limit radiation exposure to workers or any radiological impact on the environment. CNSC staff reviewed these measures, along with licensee corrective actions to prevent recurrence of the events, and found them to be satisfactory.
As part of their final, detailed reports on events, licensees are required to identify probable causes of events and propose corrective actions to prevent recurrences. In many cases, probable causes were related to workers not following procedures. As a result, the majority of measures taken by licensees to prevent recurrence related to retraining staff on procedures and emphasizing the importance of procedural adherence.
Figure 14: Reported events from 2013 to 2017, all sectors combined
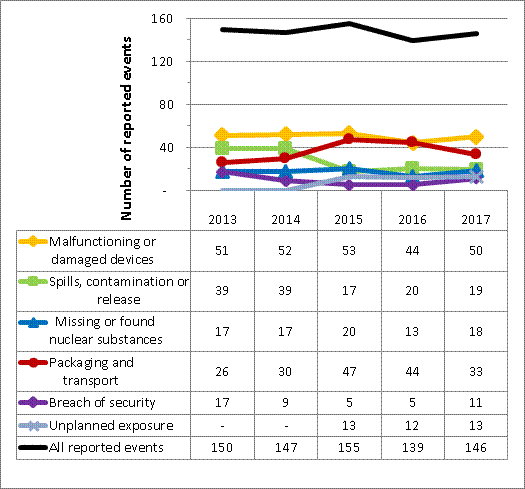
Description
| Events | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Malfunctioning or damaged devices | 51 | 52 | 53 | 44 | 50 |
| Spills, contamination or release | 39 | 39 | 17 | 20 | 19 |
| Missing or found nuclear substances | 17 | 17 | 20 | 13 | 18 |
| Packaging and transport | 26 | 30 | 47 | 44 | 33 |
| Breach of security | 17 | 9 | 5 | 5 | 11 |
| Unplanned exposure | – | – | 13 | 12 | 13 |
| All reported events | 150 | 147 | 155 | 139 | 146 |
Note: “Unplanned exposure” represents events that have led to non-routine exposures to workers or members of the public. Events of this type were covered under “breach of security” prior to 2015.
5.7.1 Malfunctioning or damaged devices
There were 50 events related to damaged or malfunctioning devices. Of these, 33 were reported as damaged devices:
-
Twenty-three cases involved damage to portable gauges:
- This number is nearly double that for 2016 and includes 17 incidents in which the portable gauges were hit or run over by vehicles or equipment at construction sites; further, a worker was injured in one of these 23 events.
- In response, CNSC staff have developed a video and user guide for portable gauge operators that emphasize the critical importance of not leaving gauges unattended and of maintaining awareness of the surroundings at all times.
- Six events involved damage to fixed gauges.
- Four events involved damage to exposure devices, often following a drop or an impact.
None of the above resulted in damage to the source or source leakage.
Seventeen events in this category corresponded to malfunctioning radiation devices:
- Eight incidents involved malfunctioning fixed gauges: in six cases, the shutters failed to close properly; in one case, the source rod could not be retracted properly; and in the remaining case, the gauge had an elevated external dose rate when left in the “off” position as a result of a manufacturing defect (a small thin spot or small hole in the shutter).
- Four incidents involved malfunctioning portable gauges, three of which had shutters stuck in the open position, and the fourth case involved a loose screw cap.
-
Four events involved malfunctioning exposure devices, two of which were related to sealed sources that could not
be retracted into the safe position, and one in which the source became disconnected from the device.
- One of these events was previously reported to the Commission in March 2018 as an EIR (event initial report) for a potential effective dose to a certified EDO over the annual limit for NEWs; additional follow-up was conducted, including a bioassay of the affected worker, and it was concluded that there was no overexposure as a result of the source disconnect and retrieval ‒ the bioassay results confirm that the dose recorded by the certified EDO’s dosimeter was a non-personal dose.
All malfunctioning devices were taken out of service as required by the Nuclear Substances and Radiation Devices Regulations and either repaired or sent for disposal.
One event corresponded to malfunctioning Class II prescribed equipment:
- A door switch at a radiation treatment bunker malfunctioned, the licensee’s proposed mitigation measures to allow continued use of the treatment areas until the malfunctioning equipment was repaired.
Any radiation exposure to a person as a result of damaged or malfunctioning devices was well below the annual public regulatory dose limit of 1 mSv. All events in this category are closed.
5.7.2 Spill or contamination
All licensees are required to document, record and investigate every skin contamination event to ensure that work practices are optimized and to minimize the probability of repeat occurrences. None of the spill, contamination or release events reported in 2017 posed a risk to the environment or resulted in members of the public receiving a dose. There were 19 events related to spills or minor contamination:
- Seven incidents involved spills of technetium-99m ‒ one such incident resulted in skin contamination of a worker below regulatory limits.
- There were six spills involving iodine-131; one such spill resulted in contamination of a worker who received a dose to the hand below regulatory limits.
- Two spills involved fluorine-18; neither spill resulted in contamination to a person.
- One incident involved a spill of gallium-68 without contamination to a person.
- There were two incidents involving a spill of sulfur-35: in one case, a non-NEW received a dose below regulatory limits to the skin of the hand and to the whole body, and in the second case, a non-NEW received a dose below regulatory limits after handling a vial that broke during transport.
- One licensee reported that a Category 5 sealed source had failed a leak test; there was no loose contamination in the storage area where the sealed source was kept.
All events in this category are closed.
5.7.3 Missing, stolen or found nuclear substances
Licensees are required to have in place physical security measures, practices and programs to prevent the loss, illegal use, illegal possession or illegal removal of nuclear substances during their entire lifecycle, including while they are in storage or during transport. Table 4 provides a summary of all events involving missing or found nuclear substances.
In 2017, there were 11 reported events involving lost or stolen nuclear substances.
- Ten events that involved lost nuclear substances consisted of Category 4 and 5 sources considered to be of low and very low risk respectively; the sealed sources or radiation devices were recovered in three of these cases.
- One event involved a portable gauge that was stolen from the back of a vehicle overnight. The police were notified immediately. The device, a Category 4 source, has not been recovered.
There were seven events involving found sources in 2017.
- A licensee employee left a sealed source used for calibration unattended within a protected area; it was found by another employee. There were no overexposures as a result of the incident.
- A licensee discovered two uranium pellets that predated their inventory; contamination or exposure was determined to be unlikely based on the activity of the pellets and the location where they were found.
-
There were five events in which nuclear substances were found in the public domain; in all cases, arrangements
were made to return or dispose of the devices properly.
- In three cases, devices containing sealed sources were discovered at scrap metal facilities: one was an unknown item containing Ra-226 and two were historical artifacts.
- In one case, a portable gauge was left at the doorstep of a member of the public.
- In another case, a fixed gauge fell through the bottom of a trailer and was found by a construction worker on the side of a highway.
Events involving lost, stolen or recovered radiation devices and sealed sources are reported in the Report on Lost or Stolen Sealed Sources and Radiation Devices, which is updated regularly.
| # | Date | Event summary | Sealed source category | INES rating level | Status |
|---|---|---|---|---|---|
| 2951 | Jan. 5 | A licensee reported finding two uranium pellets in a desk at their facility. The pellets were added the licensee’s inventory. | n/a | 0 | Found source |
| 2961 | Jan. 18 | A therapeutic source of iodine-125 was reported missing. The source was not recovered. | 5 | 0 | Not recovered (very low risk) |
| 2998 | Feb. 20 | A cesium-137 source used for calibration was reported missing. The source was not recovered. | 5 | 0 | Not recovered (very low risk) |
| 3034 | Mar. 3 | Two cesium-137 sources were reported missing. They were recovered two weeks later. | 5 | 0 | Recovered |
| 3033 | Mar. 29 | An item containing 200 MBq (megabecquerels) of radium-226 was discovered at a scrap metal facility. | 5 | 0 | Found source |
| 3054 | Apr. 26 | A portable gauge in its Type A package was left at the doorstep of a member of the public. The CNSC contracted a servicing licensee to pick up the gauge for disposal. | 4 | 0 | Found source |
| 3061 | May 7 | Two sealed sources used for well-logging fell off a truck during transport. They were recovered the same day. | 4 | 0 | Recovered |
| 3068 | May 16 | A portable gauge fell off the back of a truck during transport. It was recovered three days later by a member of the public. | 4 | 0 | Recovered |
| 3088 | Jun. 20 | Five liquid scintillation counters were reported missing. They have not been recovered. | 5 | 0 | Not recovered (very low risk) |
| 3106 | Jul. 14 | A historical artifact ‒ a cable coated with radioactive zinc sulfide ‒ was discovered at a scrap metal facility. The cable was disposed of by a consultant. | n/a | 0 | Found source |
| 3159 | Oct. 1 | A portable gauge was stolen from the back of a vehicle overnight. The device has not been recovered. | 4 | 1 | Not recovered(low risk) |
| 3164 | Oct. 5 | A sealed source (cesium-137) used for calibration was left unattended within a licensee’s protected area. | 5 | 0 | Recovered |
| 3180 | Nov. 1 | A Category 5 sealed source (cesium-137) was reported missing from a hospital. The source has not been recovered. | 5 | 0 | Not recovered (very low risk) |
| 3196 | Nov. 16 | A therapeutic dose of iodine‑125 was lost following removal from a patient. The source has not been recovered. | 5 | 0 | Not recovered (very low risk) |
| 3220 | Oct. 1 | A lead-210 sealed source (Category 5) used for teaching was lost. There is no reason to suspect theft. The source was not found. | 5 | 0 | Not recovered (very low risk) |
| 3223 | Dec. 17 | A construction site worker found a fixed gauge on the side of a highway. The licensee was informed and retrieved the gauge. | 4 | 0 | Found source |
| 3226 | Dec. 19 | Historical artifacts, radium aircraft dials, were discovered in a load of metal at a scrap metal facility. | n/a | 0 | Found source |
| 3227 | Dec. 20 | A therapeutic dose of iodine-125 was reported missing. The source has not been recovered. | 5 | 0 | Not recovered (very low risk) |
5.7.4 Breach of security
The extent of security measures required by a licensee depends upon the types of nuclear substances used and activities conducted. In 2017, 11 events were reported to the CNSC relating to breaches of security.
There were six break-ins at licensee facilities.
- One incident involved a break-in at a portable gauge licensee’s location. This was the third break-in in two years. It was determined that the room containing the portable gauges was untouched and that no gauges were stolen. CNSC staff conducted a reactive security inspection to verify implementation of corrective measures. CNSC staff also took the opportunity to meet with local RCMP.
- One incident involved a break-in at a licensee’s fabrication shop. Three unknown persons broke into the licensee’s gated perimeter. This occurred while industrial radiography work was being performed in a nearby building. No radiation devices or nuclear substances were stolen and no members of the public received a radiation dose as a result.
- Two incidents involving two separate break-ins occurred at a licensee’s facility within a one-week period. The perpetrator cut the chain-link fence and targeted vehicles on the property. The licensee’s investigation indicated that there were no attempts to break into the storage area containing radioactive sources. The licensee has increased security measures to prevent future recurrences.
- One incident involved an attempted break-in at a licensee’s location. An employee found the front door access reader damaged and a storage container broken into. The licensee’s investigation determined that there was no entry into the building and that no radioactive sources had been stolen. The targeted container was not used for the storage of nuclear substances.
- One incident involved an attempted break-in at a licensee site where the perpetrators tried to cut through the perimeter fence. The nuclear substances stored onsite were not affected. Repairs were made to the fence.
There were two incidents in which workers left radiation devices unsecured and unattended.
- One incident involved workers leaving a fixed gauge unsecured and uninstalled at the end of their shift. The gauge was discovered 2.5 hours later and was promptly installed. The shutter was closed and locked during the incident and no overexposures occurred as a result. The area where the gauge was left was not accessible to unauthorized personnel.
- One incident involved a worker leaving a portable gauge unsecured and unattended at a construction site. The gauge was discovered 10 minutes later by another employee.
There were two incidents in which the door to a controlled area was left unlocked.
- One incident involved the discovery of an open and unlocked door to a room containing an irradiator. The door was one of three barriers protecting the source and could only be unlocked by security staff. The licensee’s investigation determined that there were no attempts to breach security.
- One incident involved a door to a storage area for radioactive materials being left unlocked. The door was opened by a maintenance contractor who did not inform or receive consent from the radiation safety officer (RSO) to access the area. The RSO conducted a full inventory review and confirmed that no sources were missing.
In the final breach of security incident, an unauthorized individual drove into a licensee’s rail yard in an unmarked vehicle. The individual was escorted to security and left the site. The licensee implemented security measures to prevent similar occurrences in the future.
There was no access to, or theft of, nuclear substances or radiation devices as a result of any of these events. All of the events are closed.
5.7.5 Packaging and transport
Approximately 1 million packages containing nuclear substances are safely transported each year in Canada. In 2017, there were 33 events reported to the CNSC relating to packaging and transport.
- There were 17 incidents in which vehicles transporting nuclear substances or radiation devices were involved in collisions. None resulted in damage to the packages on board.
- Seven events involved packages that were misrouted or delayed during transport. In all cases, the packages were delivered to the proper location or returned to the originator.
- Three incidents involved packages that were damaged during transport. In all cases, the reports concluded that the nuclear substances or radiation devices were fully contained within the packages and that there was no release as a result of the incidents.
- Two events involved packages that were misclassified. In both cases, there was no impact on the health and safety of workers, the public or the environment.
- One event involved a package with external contamination after previously being used to transport isotopes used in nuclear medicine. The contamination found on the package was above regulatory limits. There was no contamination inside the vehicle used for transport or on the driver.
- One event involved a sealed source in a Type A package that was discovered to be empty upon receipt. It was later confirmed that the source had been shipped at an earlier date.
- One incident involved a spill inside a package. A full vial of a medical isotope, technetium-99m, was transported with a damaged lid. The spill was contained to the inside and no contamination was detected on the outside of the package.
- One event involved a Type A package containing a portable gauge that was not locked during transport. There were no signs of tampering with the locks.
None of the events resulted in releases to the environment or doses to members of the public above regulatory limits. All events related to packaging and transport are closed.
5.7.6 Unplanned exposures to persons
When nuclear substances are used, there may be situations that lead to unplanned exposures to persons. Often these events involve people entering restricted work areas, as is required in the industrial radiography subsector, for example.
In 2017, there were 13 events reported to the CNSC that led to unplanned exposures to persons. There was one occurrence that resulted in a NEW receiving a dose above the regulatory limits. This event was ranked as INES level 2.
- Six events involved breaches of safety barriers where workers not involved in the operation of radiation devices entered restricted areas that were established prior to the use of exposure devices. In all cases the workers received doses of between 0 and 50 µSv (microsieverts), well below the public regulatory dose limit of 1 mSv (millisievert).
- Three events involved portable gauges that were transported with open shutters. It was determined that there was no overexposure to any personnel involved, and no risk to the environment or the public as a result of these events.
- One event involved a NEW receiving skin contamination in excess of regulatory limits. The NEW in a nuclear medicine facility became contaminated after handling a contaminated cart. The worker received 2,366 mSv to the skin of the left hand and 124 mSv to the right. The regulatory limit for skin of the hand is 500 mSv. The worker was monitored following the incident and has shown no negative effects.
- One event involved contamination of a NEW who received a dose to the skin of the wrist while working with fluorine-18 without all of the necessary personal protective equipment. The exposure was below the regulatory limits.
- One event involved non-NEWs removing a fixed gauge from operation. No radiation surveys were conducted before the gauge was moved, and the shutter was left open while in storage for a month. The doses received by the non-NEWs were below the regulatory limits of 1 mSv per year.
- One event involved two NEWs who approached an exposed industrial radiography source and failed to recognize that their personal dosimetry alarms had gone off. The workers received doses below the regulatory limits for NEWs, which is 50 mSv per year.
All events in this category are closed.
5.7.7 Flood or fire
In 2017, there was one fire event and one flood event at waste nuclear substance licensee installations.
- One event involved a fire at a licensee site. The nuclear substances were safely stored outdoors and were undamaged by the fire. The Commission was informed of the event on April 11, 2017 and information regarding the event was posted to the CNSC website.
- One event involved a flood at a licensee’s warehouse and processing area after the main water supply ruptured. No radioactive materials were involved and all packages remained undamaged. Swipes and samples were taken and showed no signs of contamination.
5.7.8 International events in 2017
The CNSC monitors events reported to the International Atomic Energy Agency (IAEA) through the IAEA’s International Reporting System for Operating Experience, a database of events from around the world. For the purpose of sharing information, regulators report on a voluntary basis events that occur in their countries. Reported events are assigned a significance according to the INES scale. In 2017, one level 3 event and five level 2 events relevant to the sectors covered in this report were reported to the IAEA by nuclear regulators.
The level 3 event was as follows:
- A quality control analyst received a dose to the hands above the regulatory limits. The quality control analyst dropped a vial of molybdinum-99 in a fume cupboard. The liquid splashed on to the analyst’s gloved hands. Both pairs of gloves were contaminated. The analyst monitored their hands and found they were also contaminated. The dose received was estimated to be 850 mSv, which is above the limit for extremity doses. In the weeks following the incident, the analyst’s hands showed blistering and erythema. [reported by Australia]
The level 2 events were as follows:
- A radiopharmacy technician received a whole-body dose above the regulatory limits. The technician was working with molybdinum‑99/technitium‑99m, technitium‑99m and gallium‑68. The worker’s dosimeters reported overexposures two months in a row. The probable causes were human error and procedural non-adherence. Non-personal doses were considered but could not be confirmed. The worker was removed from work. Retraining of all employees was conducted. [reported by the U.S.]
- A package containing an unshielded 29.6 GBq iridium‑192 source was shipped by air from Cairo, Egypt, to Zurich, Switzerland, on its way to Brussels, Belgium. The dose rate from the package was measured at 2.6 mSv/h at 1 m upon arrival in Brussels, the destination. Conservative dose reconstructions indicate that up to 20 passengers on the flight from Cairo to Zurich may have received doses above the 1-mSv limit for exposure, and up to 8 passengers on the flight from Zurich to Brussels may have exceeded the public dose limit of 1 mSv. [reported by Belgium]
- An EDO received a whole-body dose in excess of the annual limit of 50 mSv. The individual was conducting industrial radiography. They approached the collimator after an exposure. The worker did not have a survey meter as they approached the collimator. The source was not completely retracted inside the exposure device. The individual received a dose of 54 mSv. [reported by the U.S.]
- A vehicle transporting an industrial radiography exposure device containing a Category 2 iridium‑192 source was stolen; the source had not been recovered at the time of reporting. [reported by Mexico]
- A package containing a source for industrial radiography was incorrectly labelled as being an empty package. As a result it was handled as general luggage during air transportation from Kenya to South Africa. When the package arrived at its final destination, its dose rate was measured at 6 mSv/h, which is in excess of the maximum allowable limit of 2 mSv/h. Based on this dose rate, members of the public who were on the flight could have received a dose above the regulatory limits. [reported by South Africa]
5.8 Effective doses to workers
A total of 53,350 workers in the five nuclear sectors covered in this report were monitored for occupational doses in 2017, 19,148 of whom were designated as NEWs.
One NEW in the medical sector exceeded the regulatory limit for extremities. See 6.3.1 for additional details.
Figure 15 shows the dose distribution for all workers in 2017. All workers who received doses above 1 mSv in 2017 were NEWs.
Figure 15: Sector-by-sector comparison of annual effective doses to all workers in 2017
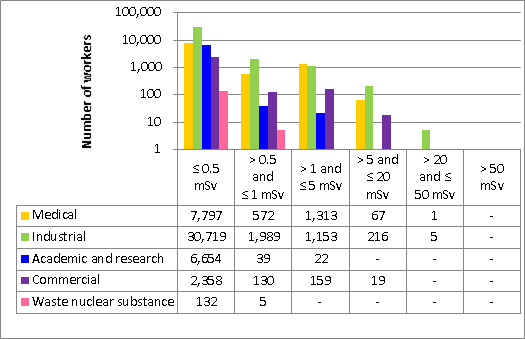
Description
| Sector | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| Medical | 7,797 | 572 | 1,313 | 67 | 1 | – |
| Industrial | 30,719 | 1,989 | 1,153 | 216 | 5 | – |
| Academic and research | 6,654 | 39 | 22 | – | – | – |
| Commercial | 2,358 | 130 | 159 | 19 | – | – |
| Waste nuclear substance | 132 | 5 | – | – | – | – |
The differences in doses to workers among sectors reflect the nature of the various activities within those sectors. Figure 15 shows the doses received by the 19,184 NEWs monitored in 2017, while figure 16 shows the doses of NEWs from 2013 to 2017.
Figure 16: Annual effective doses to NEWs, 2013–2017, all sectors combined
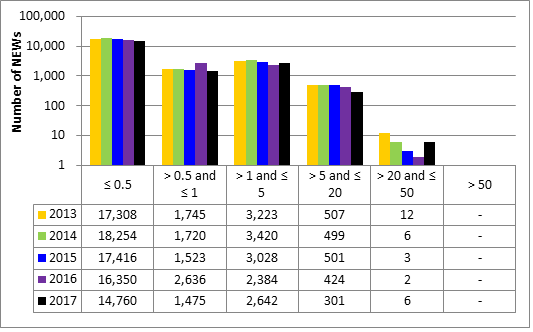
Description
| Year | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| 2013 | 17,308 | 1,745 | 3,223 | 507 | 12 | – |
| 2014 | 18,254 | 1,720 | 3,420 | 499 | 6 | – |
| 2015 | 17,416 | 1,523 | 3,028 | 501 | 3 | – |
| 2016 | 16,350 | 2,636 | 2,384 | 424 | 2 | – |
| 2017 | 14,760 | 1,475 | 2,642 | 301 | 6 | – |
Health Canada administers the National Dose Registry, which is Canada’s national repository of dose records for workers who are monitored for occupational exposure to ionizing radiation, whether under the CNSC’s mandate or not. Health Canada has published the Report on Occupational Radiation Exposures in Canada 2017, which covers the years 2007 to 2016. Doses monitored by the National Dose Registry were consistent with the doses reported by licensees to the CNSC, where comparable job categories and subsectors were examined.
6 Medical sector
Licensees in the medical sector use nuclear substances and operate accelerators and other Class II prescribed equipment for diagnostic and therapeutic purposes in hospitals and medical clinics. In 2017, this sector accounted for 457 Canadian Nuclear Safety Commission (CNSC) licences and a total of 9,750 workers, 6,706 of whom were designated as nuclear energy workers (NEWs).
The results of CNSC staff’s evaluation of the regulatory performance of all medical sector licensees inspected in 2017 are included in the overall results. The following three subsectors are highlighted in further detail:
- nuclear medicine – medium-risk activity
- radiation therapy – medium-risk activity
- veterinary nuclear medicine – medium-risk activity
Figure 17: Veterinary nuclear medicine clinic (Source: CNSC)
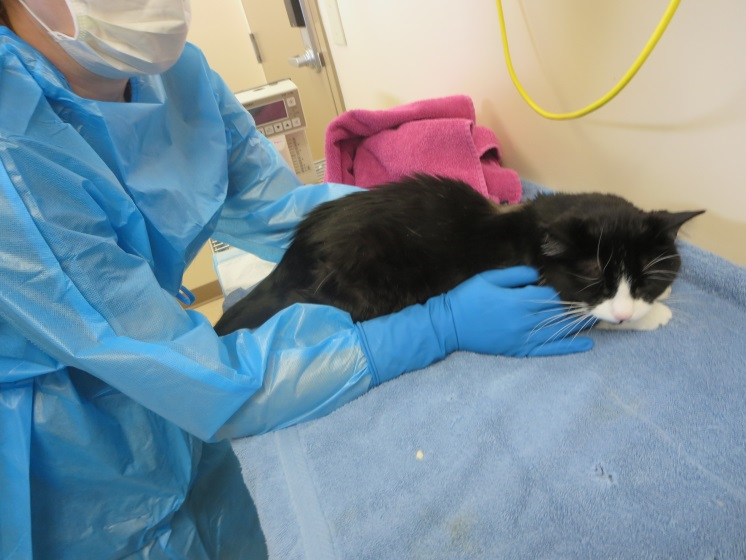
6.1 Sector overview
Medical applications using radiopharmaceuticalsFootnote 3 target specific tissues and organs, and allow for the delivery of nuclear substances to specific areas of the body for diagnostic testing or treatment.
Diagnostic nuclear medicine studies assist in the diagnosis of medical conditions based on the physiological functions of organs, tissues or bones. Radiopharmaceuticals containing nuclear substances such as technetium‑99m and fluorine‑18 are administered to patients for imaging purposes. Examples of common diagnostic nuclear medicine procedures include myocardial perfusion scans (to visualize heart function and blood flow), bone scans (to evaluate bone metabolism, infection or tumours) and renal scans (to evaluate kidney function).
Radioisotopes are also used in many therapeutic nuclear medicine procedures. For example, iodine‑131 is used to treat diseases of the thyroid gland, while other isotopes such as yttrium‑90 may be used in conjunction with antibodies for site-specific treatment of certain cancers.
Medical linear accelerators and brachytherapy equipment are also used for therapeutic procedures. These devices are used to treat cancer by delivering carefully controlled doses of radiation to cancerous tissue.
Veterinary nuclear medicine uses techniques similar to those employed in human nuclear medicine. Veterinary clinics across the country offer a wide range of diagnostic and therapeutic nuclear medicine procedures and, in some cases, radiation therapy treatment using medical accelerators.
Figure 18: CNSC inspectors examining the collimator of Class II equipment used in medical applications
(Source: CNSC)

6.2 Summary of safety assessment
Based on their evaluation and verification of licensee performance, CNSC staff concluded that the safety performance of most licensees in the medical sector was satisfactory in 2017.
Doses received by NEWs in this sector remained low, with the majority of workers receiving effective doses below 1 mSv (millisievert). One NEW received a dose to the skin of the hand above the annual limit for extremities.
Of all the licensees inspected in 2017, the majority were found to be compliant in the four safety and control areas (SCAs) covered in this report:
- 96% were compliant in management system
- 86% were compliant in operating performance
- 81% were compliant in radiation protection
- 81% were compliant in security
In cases where non-compliances were noted, licensees took appropriate corrective actions, satisfactory to CNSC staff, to address the non-compliances.
No enforcement actions were taken against licensees in the medical sector.
6.3 Safety performance measures
6.3.1 Doses to workers
NEWs in the nuclear medicine subsector continued to receive higher doses than workers in other medical subsectors as a result of directly administering nuclear substances to patients and constantly working in environments where patients are in close proximity to health professionals. The vast majority of these NEWs received doses below 5 mSv, as shown in figure 19. The doses to NEWs in the nuclear medicine subsector from 2013 to 2017 are shown in figure 20.
In 2017, one NEW in the medical sector received a dose to the skin of the hand above regulatory limits in an event ranked level 2 on the International Nuclear and Radiological Event Scale (INES). Following an investigation, it was determined that the cause of the incident was the handling of a contaminated cart. The cart had likely become contaminated when a patient removed the drinking straw used to orally administer the idodine‑131 from the shielded drinking vial, which caused non-visible contamination to spray onto the handle of the cart. After the therapies were completed, the worker touched the cart without wearing gloves, which caused a transfer of contamination to the hand. The estimated skin dose to the worker was 2,366 mSv for the right hand. The regulatory limit for extremities is 500 mSv. CNSC staff reviewed the skin dose calculation and concurred with the results. A return-to-work authorization letter was issued by a CNSC designated officer on March 17, 2017. The worker has not experienced adverse effects to the skin as a result of the radiation exposure and was closely monitored for six months following the incident. The event was presented to the Commission at the April 2017 meeting. As this was the second event involving an exposure of a worker in excess of regulatory limits at this licensee’s facilities, CNSC staff conducted a Type I inspection in 2017, which identified some program deficiencies. The licensee continues to work on implementing the corrective action plan resulting from the inspection.
Figure 19: Medical sector performance – annual effective doses to NEWs in 2017
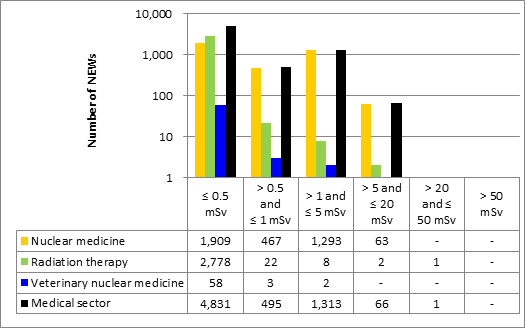
Description
| Sector | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| Nuclear medicine | 1,909 | 467 | 1,293 | 63 | – | – |
| Radiation therapy | 2,778 | 22 | 8 | 2 | 1 | – |
| Veterinary nuclear medicine | 58 | 3 | 2 | – | – | – |
| Medical sector | 4,831 | 495 | 1,313 | 66 | 1 | – |
Note: The total number of NEWs shown in the medical sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
Figure 20: Nuclear medicine subsector performance, annual effective doses to NEWs, 2013–2017
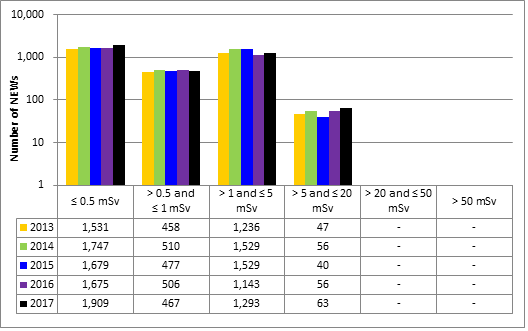
Description
| Year | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| 2013 | 1,531 | 458 | 1,236 | 47 | – | – |
| 2014 | 1,747 | 510 | 1,529 | 56 | – | – |
| 2015 | 1,679 | 477 | 1,529 | 40 | – | – |
| 2016 | 1,675 | 506 | 1,143 | 56 | – | – |
| 2017 | 1,909 | 467 | 1,293 | 63 | – | – |
6.3.2 Management system
The overall compliance rating for management system in the medical sector was 96% (106 of 110 inspections were rated satisfactory or fully satisfactory) in 2017, as shown in figure 21. This is consistent with performance in 2015 and 2016. A sector-to-subsector comparison of inspection ratings is provided in figure 22. The variability in the year-over-year ratings in the radiation therapy subsector is, in part, an artifact of the small number of inspections conducted.
The non-compliances in the management system SCA for medical licensees were using nuclear substances or possessing radiation device models that did not appear on their licences, and failing to file an annual compliance report with the CNSC according to requirements. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 21 : Medical sector performance – details of inspection ratings for management system, 2015–17
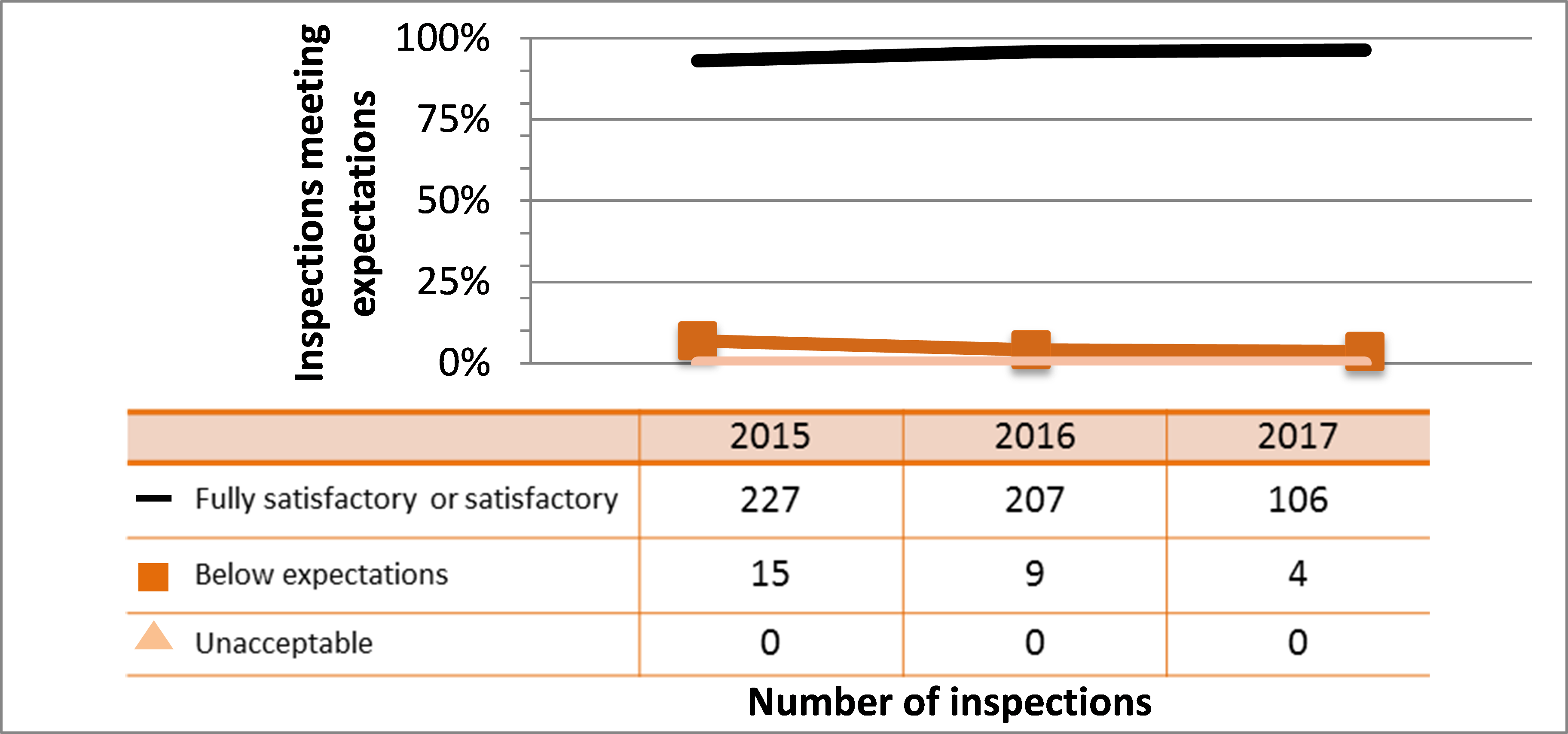
Description
| Inspection ratings | 2015 | 2016 | 2017 |
|---|---|---|---|
| Fully satisfactory or satisfactory | 227 | 207 | 106 |
| Below expectations | 15 | 9 | 4 |
| Unacceptable | 0 | 0 | 0 |
Figure 22: Medical sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for management system, 2015–17
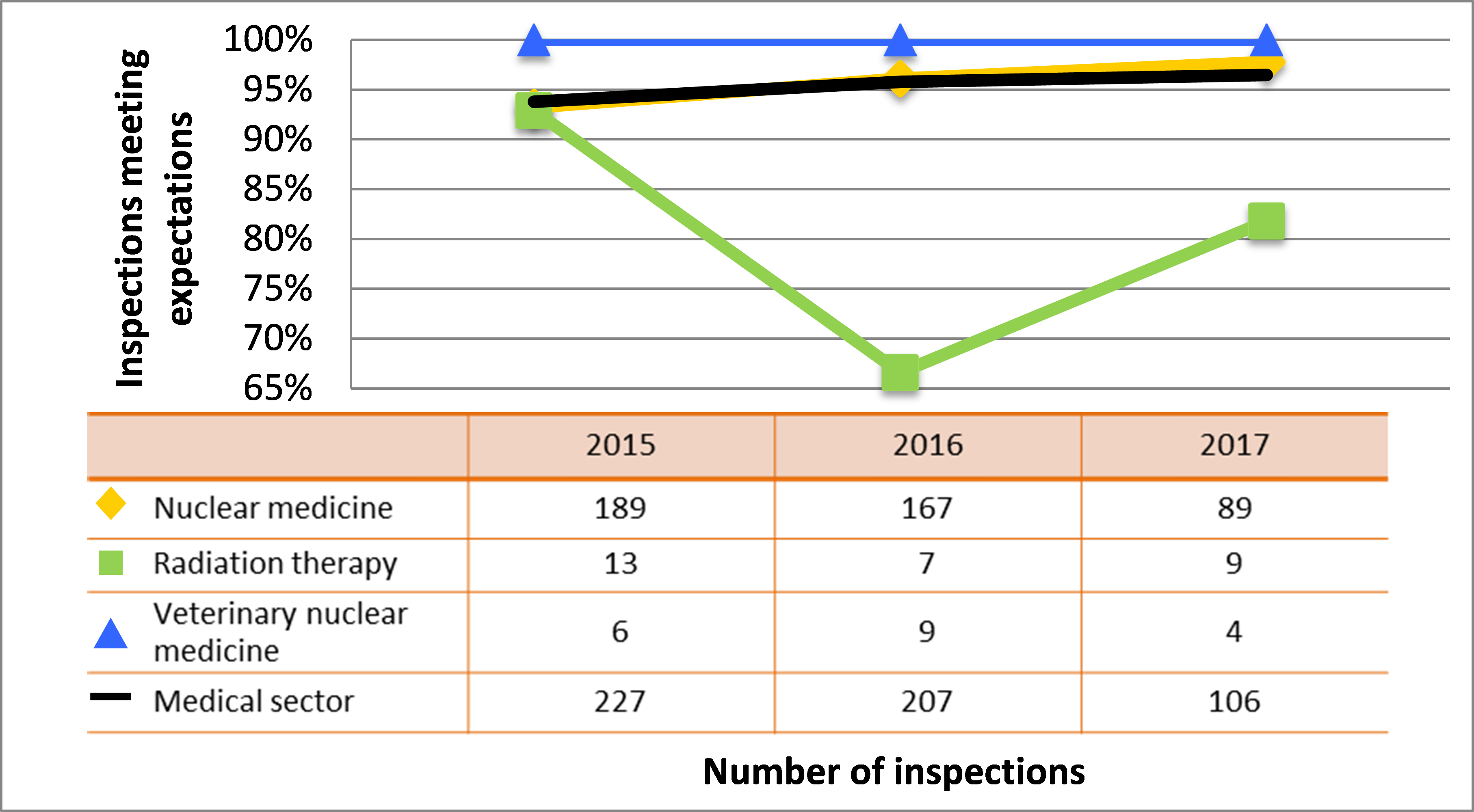
Description
| Sector | 2015 | 2016 | 2017 |
|---|---|---|---|
| Nuclear medicine | 189 | 167 | 89 |
| Radiation therapy | 13 | 7 | 9 |
| Veterinary nuclear medicine | 6 | 9 | 4 |
| Medical sector | 227 | 207 | 106 |
Note: The number of inspections shown in the medical sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
6.3.3 Operating performance
The overall compliance rating for operating performance in the medical sector was 86% (100 of 116 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 23. This is slightly lower than last year. A sector-to-subsector comparison of inspection ratings is provided in figure 24.
The most common non-compliances in the operating performance SCA were not following the procedures listed in documents appended in the licence, failure of workers to follow licensee procedures or use the equipment provided by the licensee, and failure to keep training records for employees. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 23: Medical sector performance – details of inspection ratings for operating performance, 2013‒17
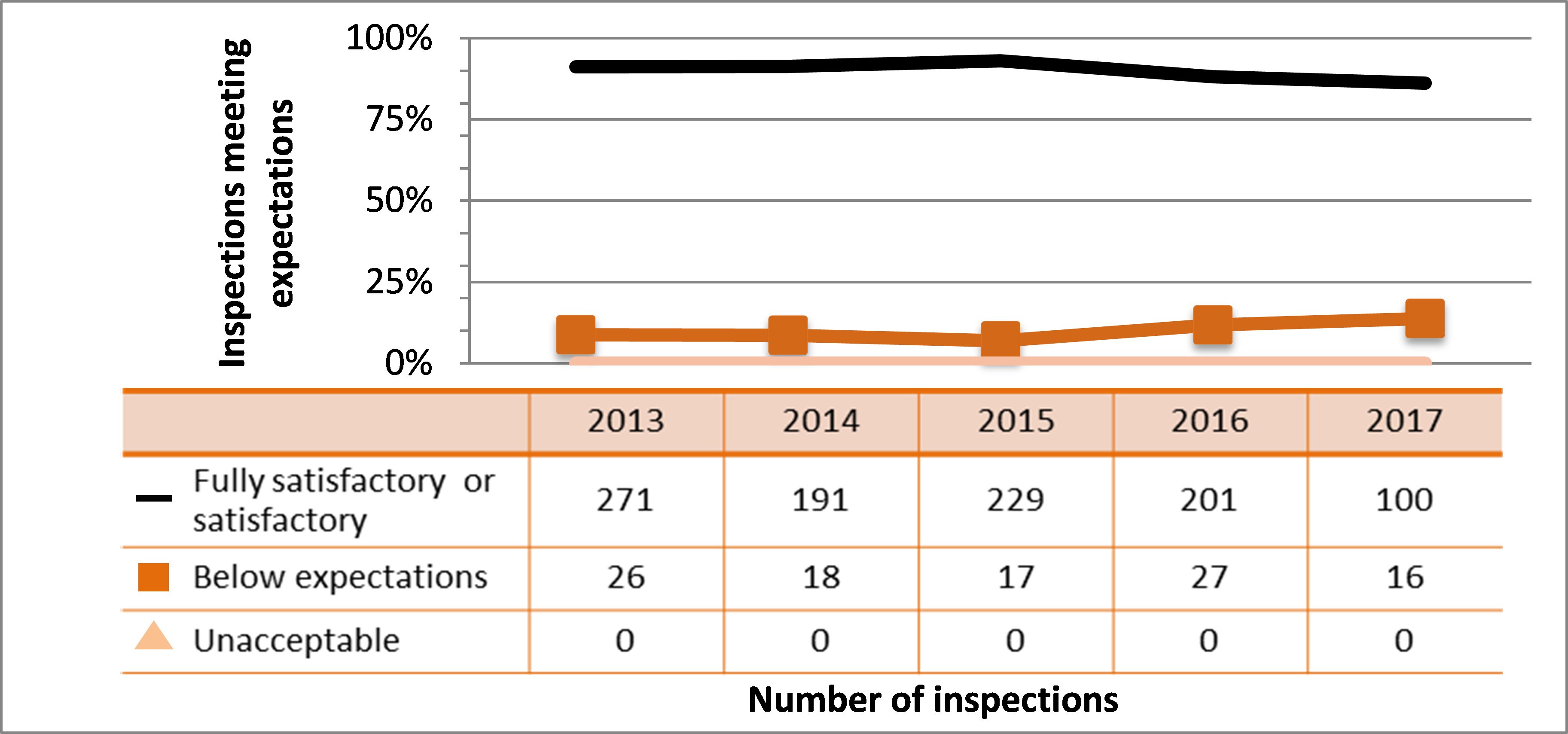
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 271 | 191 | 229 | 201 | 100 |
| Below expectations | 26 | 18 | 17 | 27 | 16 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
Figure 24: Medical sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for operating performance, 2013‒17
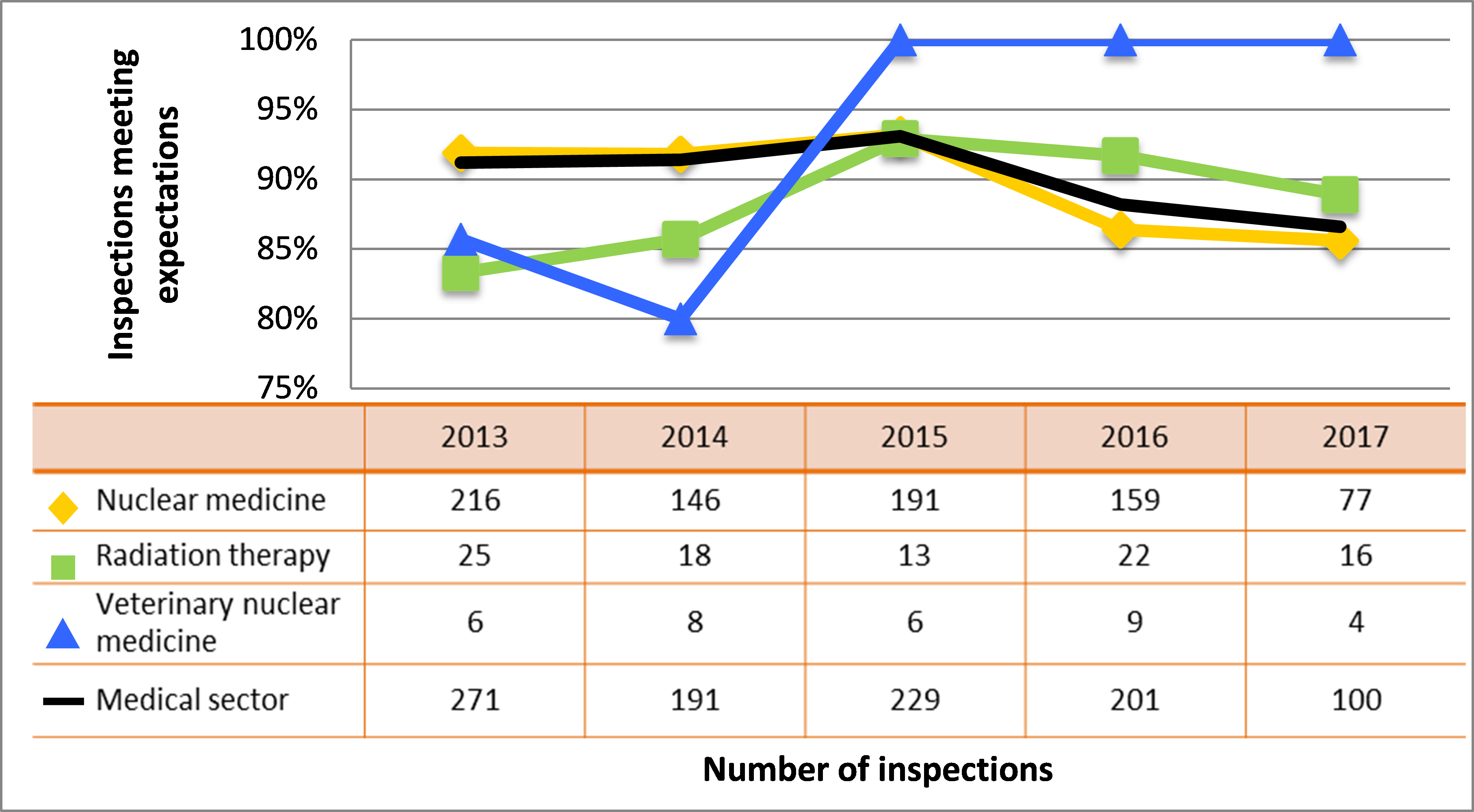
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Nuclear medicine | 216 | 146 | 191 | 159 | 77 |
| Radiation therapy | 25 | 18 | 13 | 22 | 16 |
| Veterinary nuclear medicine | 6 | 8 | 6 | 9 | 4 |
| Medical sector | 271 | 191 | 229 | 201 | 100 |
Note: The number of inspections shown in the medical sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
6.3.4 Radiation protection
The overall compliance rating for radiation protection in the medical sector was 81% (94 of 116 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 25. A sector-to-subsector comparison of inspection ratings is provided in figure 26. Compliance to requirements in the radiation protection SCA for 2017 is similar to the compliance rate for 2016, but is lower than it was in 2014 and 2015. The trend is driven primarily by lower compliance in the nuclear medicine subsector. The CNSC’s project evaluating the success factors of the radiation safety officer (RSO) and an effective radiation protection program is set to target licensees in the nuclear medicine subsector in its first phase.
The most common non-compliances for this SCA among medical sector licensees were failing to conduct thyroid monitoring within the required time frame, and failing to implement radiation programs that keep doses to workers and the public ALARA. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 25: Medical sector performance – inspection ratings for radiation protection, 2013‒17
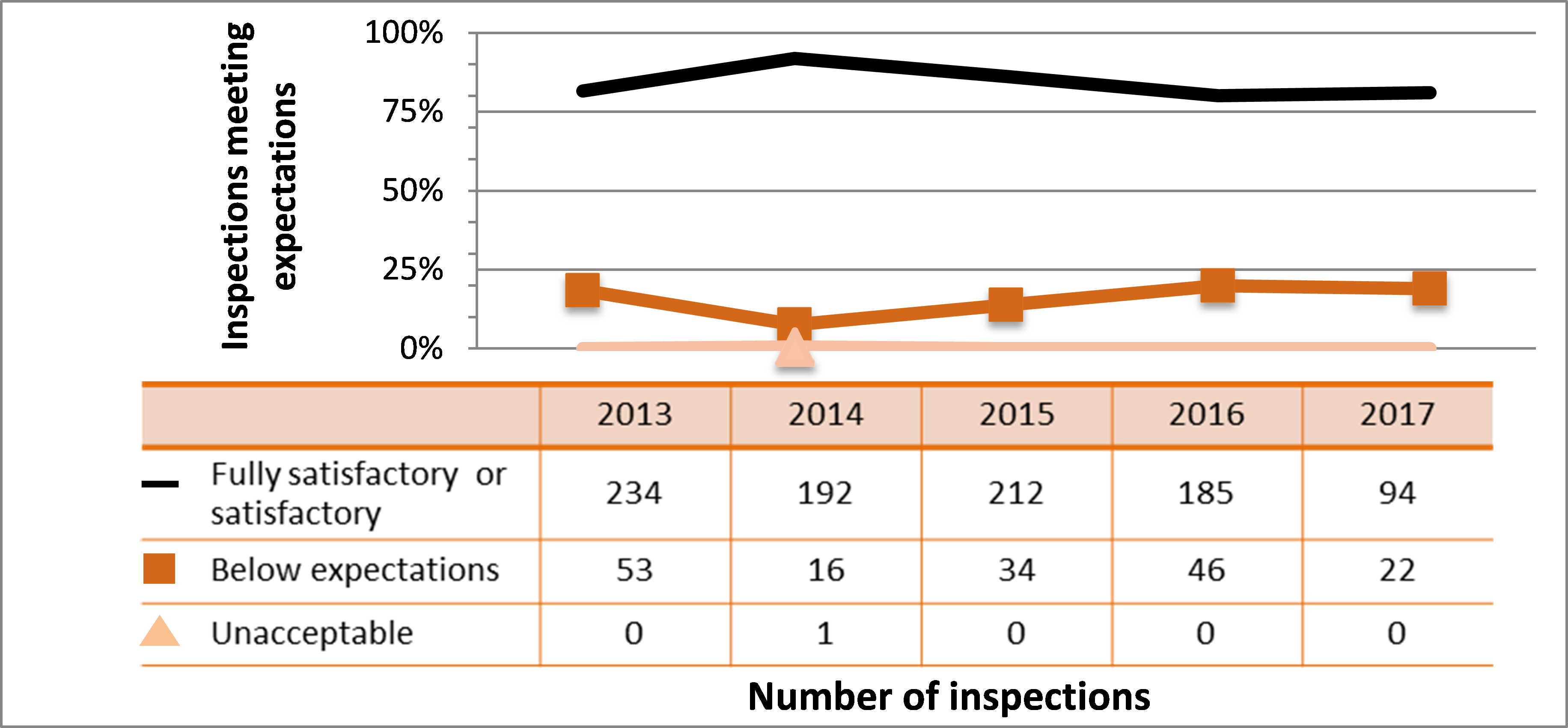
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 234 | 192 | 212 | 185 | 94 |
| Below expectations | 53 | 16 | 34 | 46 | 22 |
| Unacceptable | 0 | 1 | 0 | 0 | 0 |
Figure 26: Medical sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for radiation protection, 2013‒17
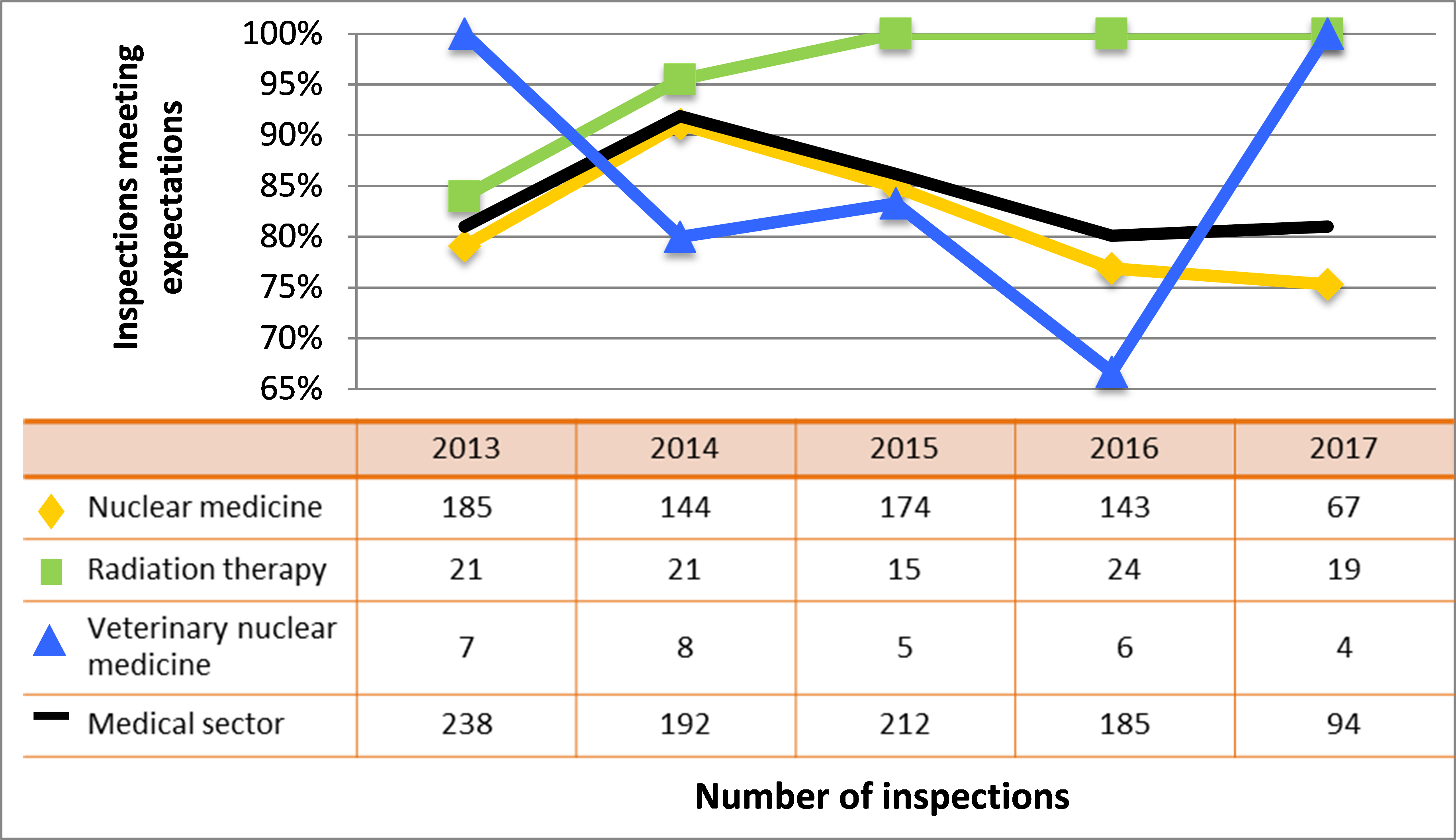
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Nuclear medicine | 185 | 144 | 174 | 143 | 67 |
| Radiation therapy | 21 | 21 | 15 | 24 | 19 |
| Veterinary nuclear medicine | 7 | 8 | 5 | 6 | 4 |
| Medical sector | 238 | 192 | 212 | 185 | 94 |
Note: The number of inspections shown in the medical sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
6.3.5 Security
The overall compliance rating for the security SCA for licensees in the medical sector was 81% (96 of 118 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 27. This continues a downward trend in the security SCA. CNSC staff continue to work with the licensees impacted by REGDOC‑2.12.3- those affected by first‑phase of implementation for Category 1 and 2 sealed sources, and also the upcoming implementation for users of Category 3, 4 and 5 sealed sources. Therefore, performance in this SCA is expected to improve once all affected licensee locations have been inspected as per the risk-informed regulatory program.
Figure 27: Medical sector performance – details of inspection ratings for security, 2014–17
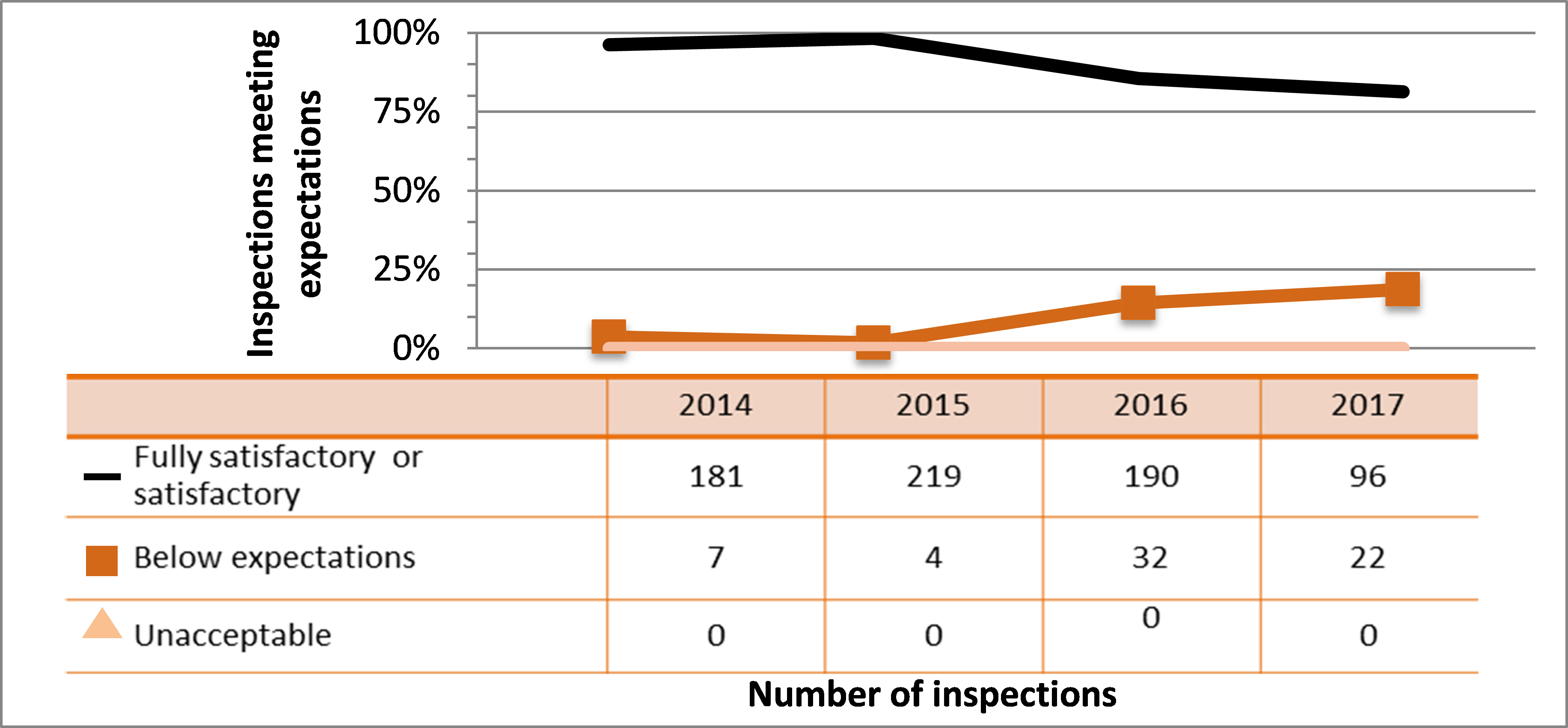
Description
| Inspection ratings | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| Fully satisfactory or satisfactory | 181 | 219 | 190 | 96 |
| Below expectations | 7 | 4 | 32 | 22 |
| Unacceptable | 0 | 0 | 0 | 0 |
7 Industrial sector
Licensees in the industrial sector use nuclear substances either in industrial facilities or as part of fieldwork or construction. In 2017, this sector accounted for 1,287 CNSC licences and a total of 34,082 workers, 7,967 of whom were designated as nuclear energy workers (NEWs).
The results of CNSC staff’s evaluation of the regulatory performance of all industrial sector licensees inspected in 2017 are included in the overall results. The following four subsectors are highlighted in further detail:
- portable gauge – medium‑risk activity
- fixed gauge – medium‑risk activity
- industrial radiography – high‑risk activity
- oil well logging – high‑risk activity
7.1 Sector overview
Typical applications of nuclear substances in the industrial sector include the measurement of physical parameters such as density, moisture content and geological composition in civil engineering. They are also used for level and flow rate in industrial facilities (such as those that support oil and gas exploration, mining and manufacturing). These nuclear substances are found in radiation devices such as fixed nuclear gauges, which monitor production processes in many industries, and portable nuclear gauges, which are often used to measure moisture and density in soil, and the compaction of asphalt in road construction.
In industrial radiography, nuclear substances are traditionally used in exposure devices for the non-destructive examination of materials. Persons operating these devices, or supervising trainees in the operation of such devices, must be certified by the Canadian Nuclear Safety Commission (CNSC). Exposure devices that are used for industrial radiography, as shown in figure 28, are engineered and operated using multiple safety barriers to reduce the potential for accidental occupational exposure. One example is dense material, such as depleted uranium, which shields users against the intense radioactivity of the source contained inside the device.
Figure 28: Exposure device used for non-destructive testing of materials (Source: CNSC)

Industrial applications of nuclear substances are as varied as the processes to which they are applied. Specific radioisotopes are chosen based on the type of radiation they emit, the intensity of their radiation and the intended application. For example, the nuclear substance chosen for industrial radiography depends on the size and density of the material to be imaged. The most common isotopes in use are iridium‑192 and selenium‑75, as well as cobalt‑60, with its high-energy gamma radiation, which is used for large structures and dense materials such as structural concrete. More recently, a small number of licensees are turning to linear accelerators for radiography imaging. This equipment facilitates the analysis of materials that are too thick to analyze using more traditional methods. Moreover, high-energy CT machines are being used to create three-dimensional images of the interior of materials such as logs and engineered wood products.
Cesium‑137, another gamma emitter, is most commonly used in portable and fixed gauges to measure density. In other industrial uses, such as measuring moisture content, portable gauges most commonly use neutron-emitting nuclear substances such as americium‑241/beryllium.
7.2 Summary of safety assessment
Based on their evaluation and verification of licensee performance, CNSC staff concluded that the safety performance of most licensees in the industrial sector was satisfactory in 2017.
Doses received by NEWs in this sector remained low, with the majority of workers receiving doses below 1 millisievert (mSv).
Of all the licensees inspected in 2017, the majority were found to be compliant in the four safety and control areas (SCAs) covered in this report:
- 98% were compliant in management system
- 82% were compliant in operating performance
- 84% were compliant in radiation protection
- 91% were compliant in security
In cases where non-compliances were noted, licensees took appropriate corrective actions, satisfactory to CNSC staff, to address the non-compliances.
The CNSC took 23 escalated enforcement actions (18 orders and 5 administrative monetary penalties (AMPs)) against licensees in the industrial sector in 2017. Further details are provided in section 7.3.6.
7.3 Safety performance measures
7.3.1 Doses to workers
NEWs in the industrial radiography subsector continued to receive higher doses than workers in other industrial subsectors, as shown in figure 29. This is a result of working in close proximity to exposure devices containing high activity sealed sources. The doses to NEWS in the industrial radiography subsector from 2013 to 2017 are shown in figure 30.
Figure 29: Industrial sector performance – annual effective doses to NEWs in 2017
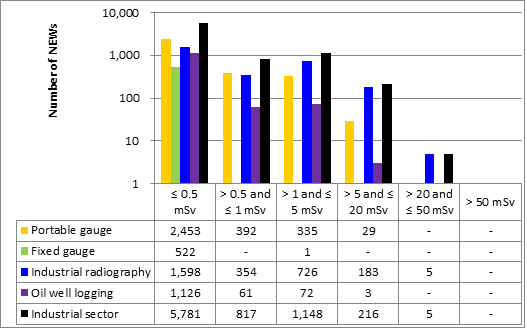
Description
| Industrial sector | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| Portable gauge | 2,453 | 392 | 335 | 29 | – | – |
| Fixed gauge | 522 | – | 1 | – | – | – |
| Industrial radiography | 1,598 | 354 | 726 | 183 | 5 | – |
| Oil well logging | 1,126 | 61 | 72 | 3 | – | – |
| Industrial sector | 5,781 | 817 | 1,148 | 216 | 5 | – |
Note: The total number of NEWs shown in the industrial sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
Figure 30: Industrial radiography subsector performance – annual effective doses to NEWs, 2013‒17
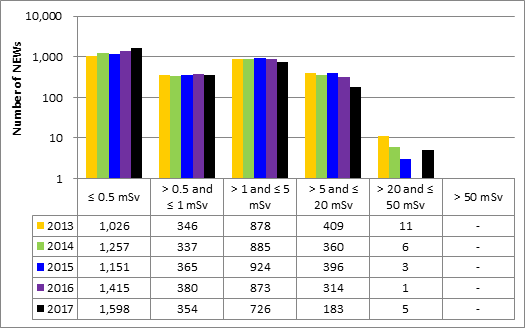
Description
| Year | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| 2013 | 1,026 | 346 | 878 | 409 | 11 | – |
| 2014 | 1,257 | 337 | 885 | 360 | 6 | – |
| 2015 | 1,151 | 365 | 924 | 396 | 3 | – |
| 2016 | 1,415 | 380 | 873 | 314 | 1 | – |
| 2017 | 1,598 | 354 | 726 | 183 | 5 | – |
7.3.2 Management system
The overall compliance rating for management system in the industrial sector was 98% (605 of 620 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 31. This is similar to previous years. A sector-to-subsector comparison of inspection ratings is provided in figure 32. There is a notable year-over-year drop in the performance of fixed gauge licensees. However, overall, more than 93% of the licensees in the industrial sector had inspections with fully satisfactory or satisfactory ratings in this SCA.
One licensee, an industrial radiography licensee, received an unacceptable rating in the management system SCA. The same inspection also yielded an unacceptable rating in the radiation protection SCA. The CNSC inspector issued an order as a result. Details of enforcement actions can be found in section 7.3.6 and appendix B.
Among industrial sector licensees, the main non-compliances for this SCA were failure of licensees to have the required records at temporary locations used for more than 90 days, failure to submit an annual compliance report to the CNSC as required, and failure to notify the CNSC of locations where they conducted licensed activities for more than 90 days.
Figure 31: Industrial sector performance – details of inspection ratings for management system, 2015–17
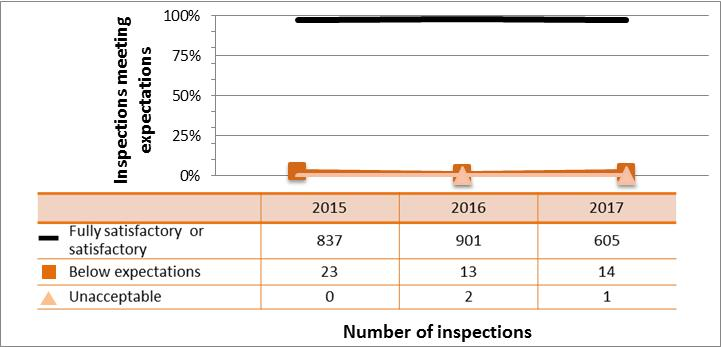
Description
| Inspection ratings | 2015 | 2016 | 2017 |
|---|---|---|---|
| Fully satisfactory or satisfactory | 837 | 901 | 605 |
| Below expectations | 23 | 13 | 14 |
| Unacceptable | 0 | 2 | 1 |
Figure 32 : Industrial sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for management system, 2015–17
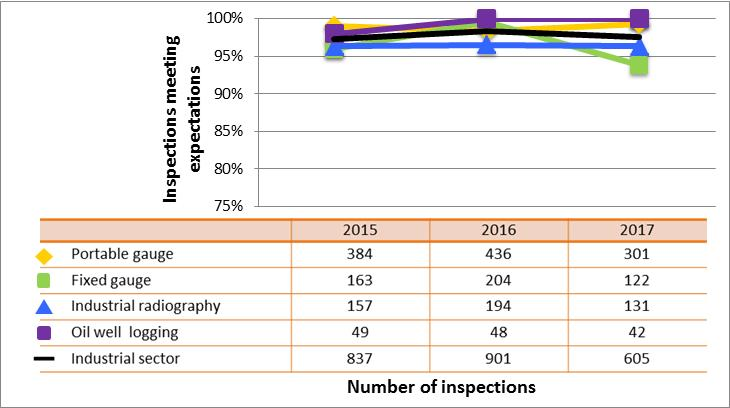
Description
| Sector | 2015 | 2016 | 2017 |
|---|---|---|---|
| Portable gauge | 384 | 436 | 301 |
| Fixed gauge | 163 | 204 | 122 |
| Industrial radiography | 157 | 194 | 131 |
| Oil well logging | 49 | 48 | 42 |
| Industrial sector | 837 | 901 | 605 |
Note: The number of inspections shown in the industrial sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
7.3.3 Operating performance
The overall compliance rating for operating performance in the industrial sector was 82% (511 of 625 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 33. Eight licensees received unacceptable ratings for operational performance. These inspections also had unacceptable ratings for the security SCA. All licensees involved were portable gauge licensees. CNSC inspectors issued orders in all eight cases. The circumstances of each are listed in section 7.3.6 and in appendix B.
A sector-to-subsector comparison of inspection ratings is provided in figure 34. The oil well logging subsector, a high-risk activity, continued to improve performance in this SCA. This is a trend that began in 2015.
Conversely, performance of licensees in the portable gauge and fixed gauge subsectors continued a downward trend. This is influenced in part by the bias imparted by inspecting licensees with poor performance at an increased frequency.
Portable gauge licensees often employ seasonal workers and thus face significant turnover in their staff. The CNSC is developing additional tools to assist portable gauge users in understanding their responsibilities as licensees and workers. A continued focus on field inspections within this sector allows CNSC staff to inspect the individuals working with the gauges. Many common items of non-compliance related to operating performance were identified during field inspections, including failure to follow procedures and failure to use the safety equipment provided.
Among fixed gauge licensees, failure to meet the licence conditions associated with vessel or hopper entry was the third-most-common item of non-compliance. In 2017, fixed gauge licensees with the licence condition for vessel or hopper entry were targeted for inspection. It is expected that poor performance against this licence condition will continue for the next few years until all licensees with the revised vessel entry licence condition on their licence have been inspected.
The most common non-compliances for this SCA were workers’ failure to follow the licensee’s procedures and to use the safety equipment provided to them, and licensees’ failure to follow the procedures in their radiation safety manuals and to keep required training records. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 33: Industrial sector performance – details of inspection ratings for operating performance, 2013–17
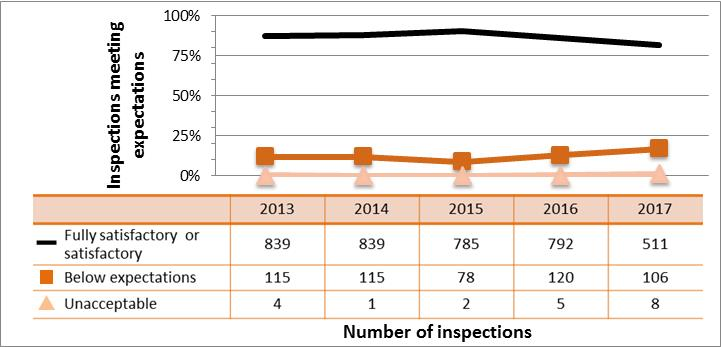
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 839 | 839 | 785 | 792 | 511 |
| Below expectations | 115 | 115 | 78 | 120 | 106 |
| Unacceptable | 4 | 1 | 2 | 5 | 8 |
Figure 34 : Industrial sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for operating performance, 2013‒17
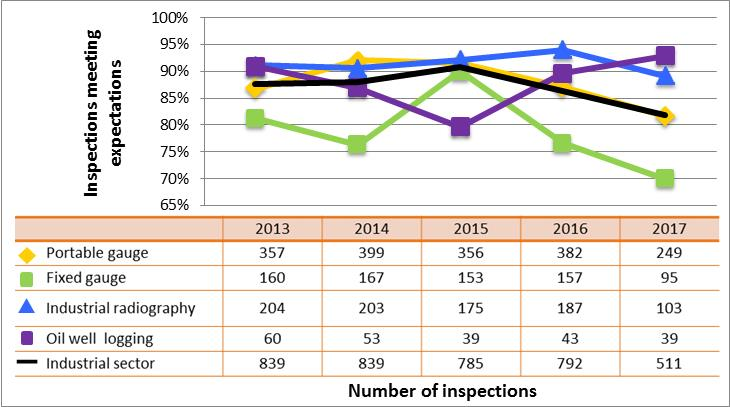
Description
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Portable gauge | 357 | 399 | 356 | 382 | 249 |
| Fixed gauge | 160 | 167 | 153 | 157 | 95 |
| Industrial radiography | 204 | 203 | 175 | 187 | 103 |
| Oil well logging | 60 | 53 | 39 | 43 | 39 |
| Industrial sector | 839 | 839 | 785 | 792 | 511 |
Note: The number of inspections shown in the industrial sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
7.3.4 Radiation protection
The overall compliance rating for radiation protection in the industrial sector was 84% (518 of 620 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 35. This is the lowest rating for this SCA since 2013. A sector-to-subsector comparison of inspection ratings is provided in figure 36. The portable gauge subsector continued to trend downward for compliance in the radiation protection SCA. As described in section 7.3.3, the CNSC is developing tools to assist portable gauge users in understanding their responsibilities as licensees and workers.
Three licensees from the industrial sector received unacceptable ratings in the radiation protection SCA. The licensees were from the portable gauge, fixed gauge and industrial radiography subsectors. CNSC inspectors issued orders to each of these licensees. The circumstances of each situation can be found in section 7.3.6 and appendix B.
The most common non-compliances for this SCA were inadequate implementation of radiation protection programs that keep doses to workers and the public ALARA, failure of licensees to post radiation warning signs, and failure to have available a calibrated survey meter. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 35: Industrial sector performance – inspection ratings for radiation protection, 2013‒17
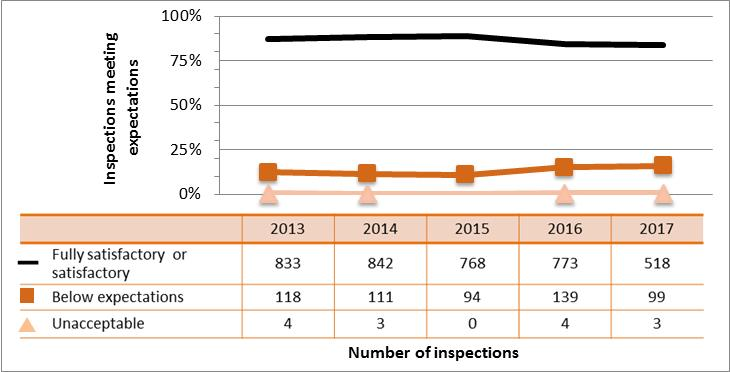
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 833 | 842 | 768 | 773 | 518 |
| Below expectations | 118 | 111 | 94 | 139 | 99 |
| Unacceptable | 4 | 3 | 0 | 4 | 3 |
Figure 36: Industrial sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for radiation protection, 2013‒17
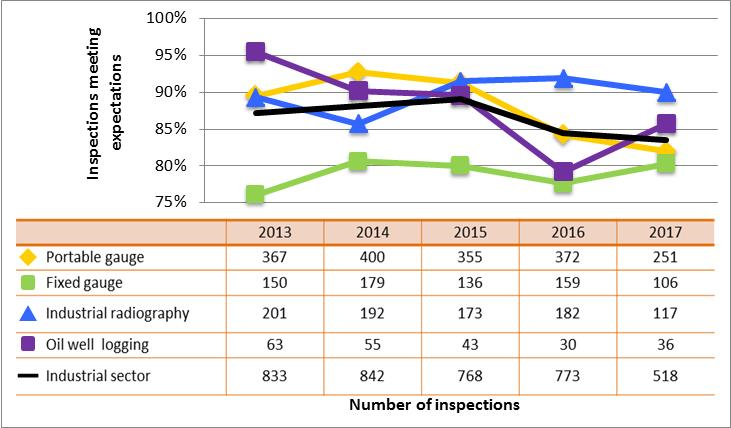
Description
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Portable gauge | 367 | 400 | 355 | 372 | 251 |
| Fixed gauge | 150 | 179 | 136 | 159 | 106 |
| Industrial radiography | 201 | 192 | 173 | 182 | 117 |
| Oil well logging | 63 | 55 | 43 | 30 | 36 |
| Industrial sector | 833 | 842 | 768 | 773 | 518 |
Note: The number of inspections shown in the industrial sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
7.3.5 Security
The overall compliance rating for the security SCA for licensees in the industrial sector was 91% (552 of 610 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 37. This is a drop in performance compared to previous years. This drop is driven primarily by decreased performance in the security SCA among portable gauge licensees, particularly non-compliances of leaving gauges unattended at work sites. Security of portable gauges was the topic of an article in the Fall 2017 edition of the DNSR Newsletter. It is also addressed in the new video and booklet described in section 7.3.3.
Nine licensees, all portable gauge licensees, received unacceptable ratings in the security SCA. CNSC inspectors issued orders in all cases. Details about the orders can be found in section 7.3.6 and appendix B.
Figure 37: Industrial sector performance – details of inspection ratings for security, 2014–17
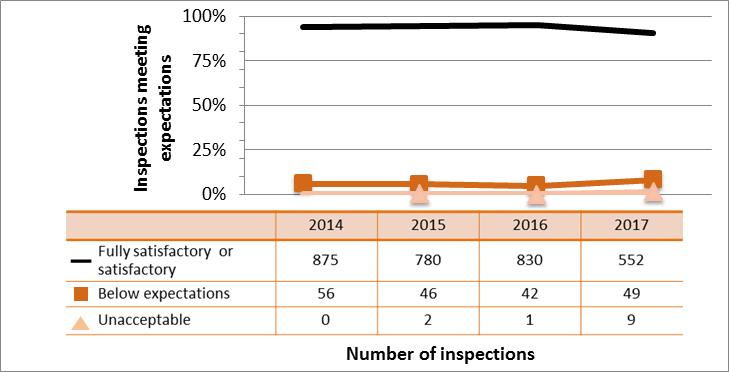
Description
| Inspection ratings | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| Fully satisfactory or satisfactory | 875 | 780 | 830 | 552 |
| Below expectations | 56 | 46 | 42 | 49 |
| Unacceptable | 0 | 2 | 1 | 9 |
7.3.6 Enforcement actions
The CNSC took 23 escalated enforcement actions against licensees in the industrial sector in 2017. These consisted of 18 orders and 5 administrative monetary penalties (AMPs). A breakdown of orders issued to different subsectors is shown in figure 38.
Figure 38: Summary of orders in the industrial sector, 2013‒17
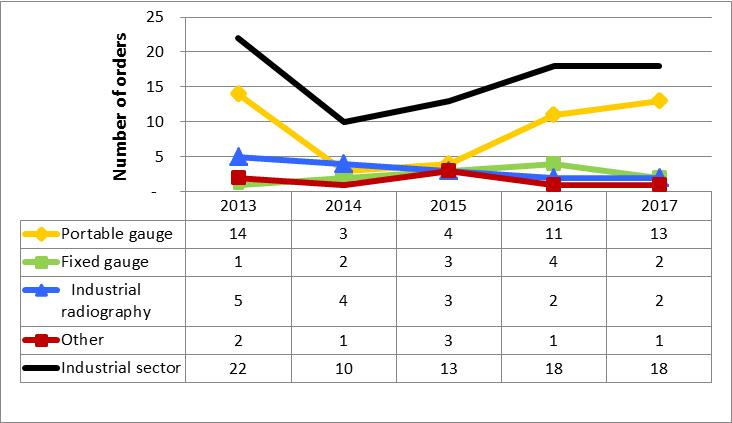
Description
| Industrial sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Portable gauge | 14 | 3 | 4 | 11 | 13 |
| Fixed gauge | 1 | 2 | 3 | 4 | 2 |
| Industrial radiography | 5 | 4 | 3 | 1 | 1 |
| Other | 2 | 1 | 3 | 1 | 1 |
| Industrial sector | 22 | 10 | 13 | 18 | 18 |
The CNSC issued 13 orders against licensees in the portable gauge subsector. All orders issued to portable gauge licensees have been addressed to the satisfaction of CNSC staff and are considered closed. The number of orders issued to the portable gauge subsector is a significant increase over past years. The majority of the orders (10 out of 13) were issued to licensees whose employees left the portable gauges unattended at work sites. CNSC staff have published various communication products aimed at highlighting the issue among portable gauge workers and improving performance in the portable gauge subsector, including the importance of maintaining security of the gauges at the work site. These products include an article in the 2017 Fall edition of the DNSR newsletter, and a user booklet and information video, available in 2018.
The CNSC issued two AMPs to licensees in the fixed gauge sector. In both cases, the CNSC determined that workers were performing vessel entry procedures without following the safety requirements prescribed by the CNSC in their licence conditions.
CNSC inspectors issued two orders to fixed gauge licensees. One was an order to prevent a fixed gauge licensee from performing vessel entries until their procedures conformed to the requirements. The second was issued to a licensee with serious non-compliances identified during an inspection. The licensee was prevented from using or transporting radiation devices until CNSC staff determined there was sufficient management control of the radiation protection program in place. Licensees met the terms and conditions of the orders. CNSC staff reviewed the corrective actions and determined they were satisfactory. The orders are considered closed.
CNSC inspectors issued two orders to licensees in the industrial radiography sector. One order was issued to a licensee when it was determined during an inspection that exposure device operators (EDOs) were conducting radiography without all of the required personal dosimetry. The order prevented any EDO or trainee from operating an exposure device until they have been assigned the required personal dosimetry and been retrained, and the licensee has submitted corrective measures for preventing recurrence of the non-compliance. The second order was issued to a licensee due to a lack of direct supervision of a trainee. The order prevented an individual from supervising trainees until the licensee was able to demonstrate that effective supervision practices were in place. An AMP was issued to the exposure device operator subject to the order. The penalty has been paid. The terms and conditions of both orders issued to industrial radiography licensees have been met to the satisfaction of CNSC staff. The orders are considered closed.
The CNSC issued an AMP to another individual from the industrial radiography sector for failure to secure an exposure device to a vehicle. The penalty has been paid.
The final order issued to licensees in the industrial sector was issued by a designated officer to a licensee whose employee transferred an X-ray fluorescence radiation device to a person not authorized by the CNSC to possess the device. Possession and use of X-ray fluorescence radiation devices is considered a low-risk activity. The order required the licensee to recover the device and transfer it to a person authorized to possess it. The licence was revoked after the device was properly transferred to another licensee. The order is considered closed.
Details of all enforcement actions issued in 2017 are provided in figure 13 and appendix B. Further information on regulatory actions, including escalated enforcement actions taken by the CNSC, is available on the CNSC website.
8 Academic and research sector
Licensed activities in the academic and research sector are conducted in universities, colleges and research laboratories. In 2017, this sector accounted for 195 licences and a total of 6,715 workers, 2,640 of whom were designated as nuclear energy workers (NEWs).
Safety performance results are provided for all licensees included in the academic and research sector, with the laboratory studies and consolidated uses of nuclear substances subsectors highlighted in further detail.
Figure 39: Example of an unsealed nuclear substance (Source: CNSC)
8.1 Sector overview
This sector focuses mainly on biological and biomedical research that primarily uses open (unsealed) nuclear substances. The sector also uses sealed sources, radiation devices and accelerators for teaching as well as for pure and applied research.
Laboratory studies and consolidated uses of nuclear substances are grouped together for the purposes of reporting here. Both are considered medium-risk activities.
CNSC laboratory
As part of its regulatory functions, the Canadian Nuclear Safety Commission (CNSC) conducts certain activities regulated under the Nuclear Safety and Control Act (NSCA). To ensure oversight transparency, CNSC management has separated the organization’s work as a licensee (which resides within the Technical Support Branch) from its work as a regulator (under the responsibility of the Regulatory Operations Branch).
The CNSC laboratory provides calibration services and analytical services for CNSC staff, including CNSC inspectors. To provide these services, the CNSC holds two licences: one for its gamma calibration irradiator located at its laboratory in Ottawa, and a second for consolidated uses of nuclear substances that covers all other activities conducted by the CNSC at its laboratory or elsewhere in Canada. Both licences were issued in accordance with the NSCA and are regulated using the same licensing and compliance verification processes that would apply to other, similar licensees.
In this report, the CNSC laboratory is included in the laboratory studies and consolidated use of nuclear substances subsector.
Doses received by NEWs working at the CNSC laboratory remained very low, with all workers receiving doses below 0.1 mSv (millisievert).
In March 2017, CNSC staff conducted an inspection at the CNSC laboratory. The inspection focused on packaging and transportation requirements. No items of non-compliance were identified during the inspection.
8.2 Summary of safety assessment
The academic and research sector continued to show satisfactory safety performance in 2017.
Doses received by NEWs in this sector remained very low, with the majority of workers receiving doses below 1 mSv.
Of all the licensees inspected in 2017, the majority were found to be compliant in the four safety and control areas (SCAs) covered in this report:
- 97% were compliant in management system
- 97% were compliant in operating performance
- 93% were compliant in radiation protection
- 96% were compliant in security
In cases where non-compliances were noted, licensees took appropriate corrective actions, satisfactory to CNSC staff, to address the non-compliances.
No enforcement actions were taken against licensees in the academic and research sector in 2017.
8.3 Sector performance measures
8.3.1 Doses to workers
Doses received by NEWs in this sector remained very low. The majority of workers received doses below 1 mSv, as shown in figure 40.
Figure 40: Academic and research sector performance – annual effective doses to NEWs in 2017
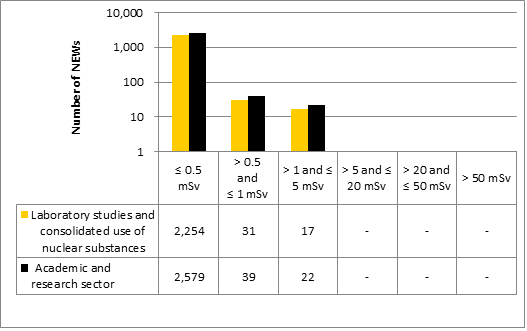
Description
| Sector | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| Laboratory studies and consolidated use of nuclear substances | 2,254 | 31 | 17 | – | – | – |
| Academic and research sector | 2,579 | 39 | 22 | – | – | – |
Note: The total number of NEWs shown in the academic and research sector row is the aggregate for the entire sector, including subsectors not highlighted in this report.
8.3.2 Management system
The overall compliance rating for management system in the academic and research sector was 97% (71 of 73 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 41. No licensees received unacceptable ratings in this SCA. A sector-to-subsector comparison of inspection ratings is shown in figure 42. Performance in 2017 was consistent with previous years.
Figure 41: Academic and research sector performance – details of inspection ratings for management system, 2015‒17
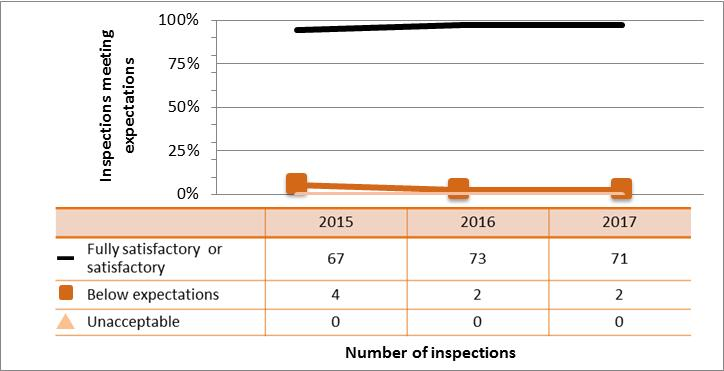
Description
| Inspection rating | 2015 | 2016 | 2017 |
|---|---|---|---|
| Fully satisfactory or satisfactory | 67 | 73 | 71 |
| Below expectations | 4 | 2 | 2 |
| Unacceptable | 0 | 0 | 0 |
Figure 42: Academic and research sector performance comparison with the laboratory studies and consolidated use of nuclear substances subsector – inspection ratings meeting or exceeding expectations for management system, 2015‒17
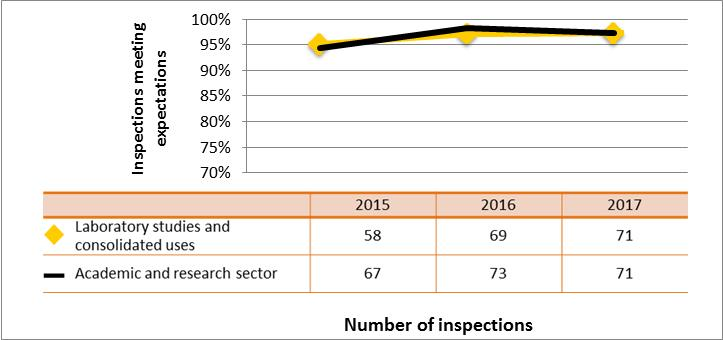
Description
| Sector | 2015 | 2016 | 2017 |
|---|---|---|---|
| Laboratory studies and consolidated use of nuclear substances | 58 | 69 | 71 |
| Academic and research sector | 67 | 73 | 71 |
Note: The number of inspections shown in the academic and research row is the aggregate for the entire sector, including subsectors not highlighted in this report.
8.3.3 Operating performance
The overall compliance rating for operating performance in the academic and research sector was 97% (73 of 75 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 43. This continues the upward trend since a low in 2015 when only 77% of inspected licensees were compliant with requirements in this SCA. A sector-to-subsector comparison of operating performance ratings is provided in figure 44.
In 2014, the CNSC inspection program for the laboratory studies and consolidated uses of nuclear substances subsector was revised based on the positive safety performance ratings and the low-risk level associated with these licensed activities. The frequency of CNSC inspections of consolidated use licensees was changed from annually to every two years, which is reflected in the decrease in the number of inspections conducted since 2014 for this subsector.
Figure 43: Academic and research sector performance – details of inspection ratings for operating performance, 2013‒17
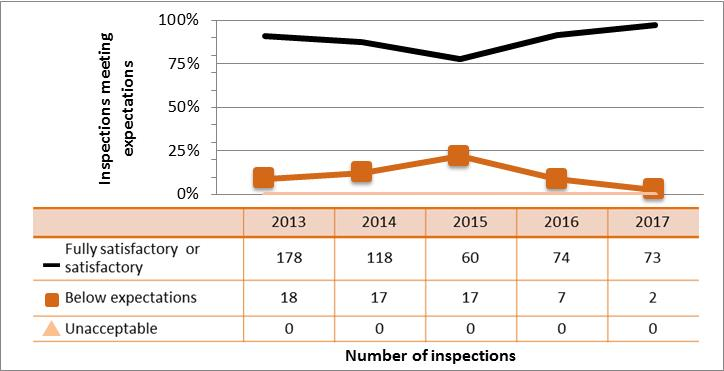
Description
| Inspection rating | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 178 | 118 | 60 | 74 | 73 |
| Below expectations | 18 | 17 | 17 | 7 | 2 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
Figure 44: Academic and research sector performance comparison with the laboratory studies and consolidated use of nuclear substances subsector – inspection ratings meeting or exceeding expectations for operating performance, 2013‒17
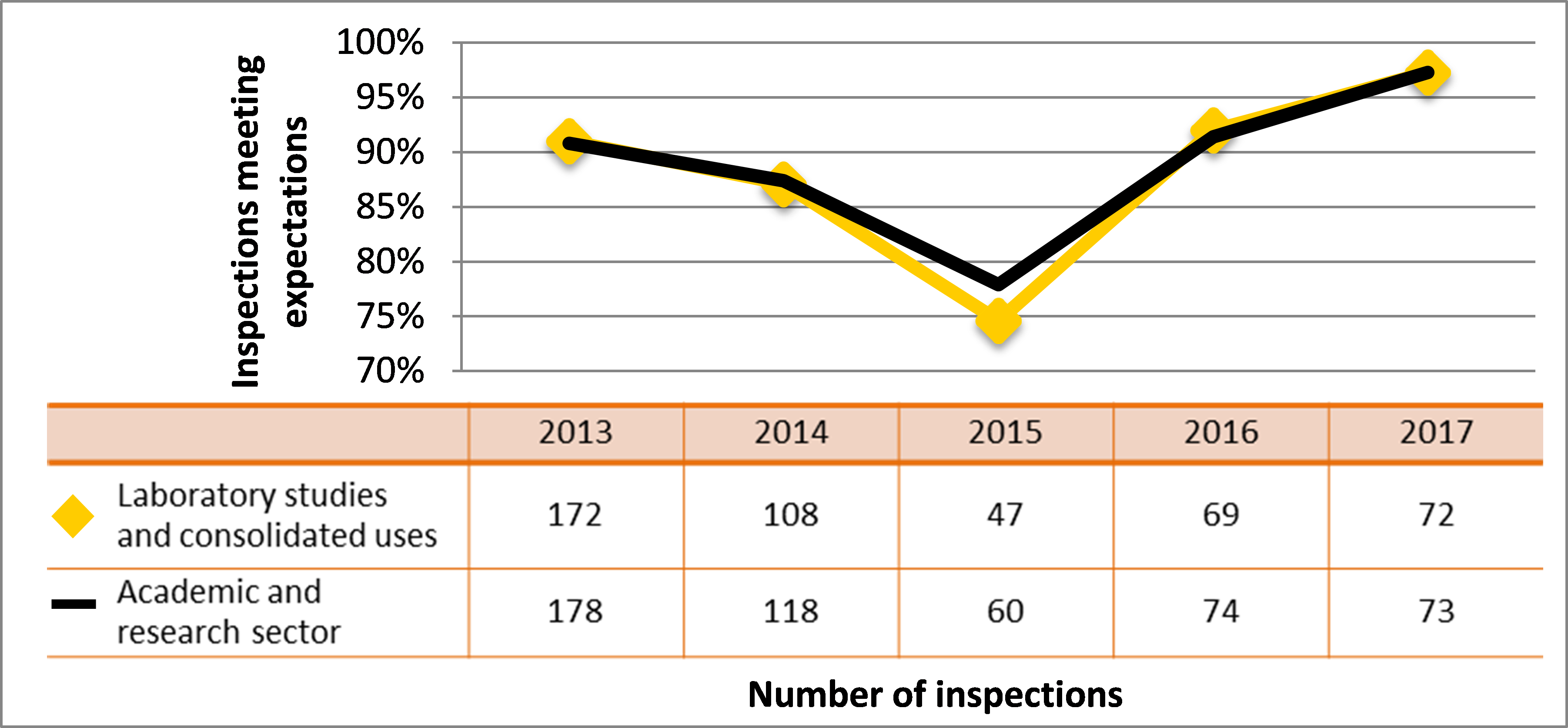
Description
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Laboratory studies and consolidated uses | 172 | 108 | 47 | 69 | 72 |
| Academic and research sector | 178 | 118 | 60 | 74 | 73 |
Note: The number of inspections shown in the academic and research row is the aggregate for the entire sector, including subsectors not highlighted in this report.
8.3.4 Radiation protection
The overall compliance rating for radiation protection in the academic and research sector was 93% (69 of 74 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 45. A sector-to-subsector comparison of inspection ratings is provided in figure 46. Performance in this SCA continues to improve.
Figure 45: Academic and research sector performance – inspection ratings for radiation protection, 2013‒17
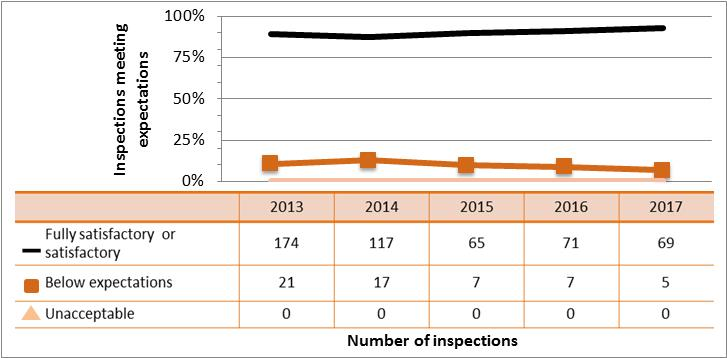
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 174 | 117 | 65 | 71 | 69 |
| Below expectations | 21 | 17 | 7 | 7 | 5 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
Figure 46 : Academic and research sector performance comparison with the laboratory studies and consolidated use of nuclear substances subsector – inspection ratings meeting or exceeding expectations for radiation protection, 2013‒17
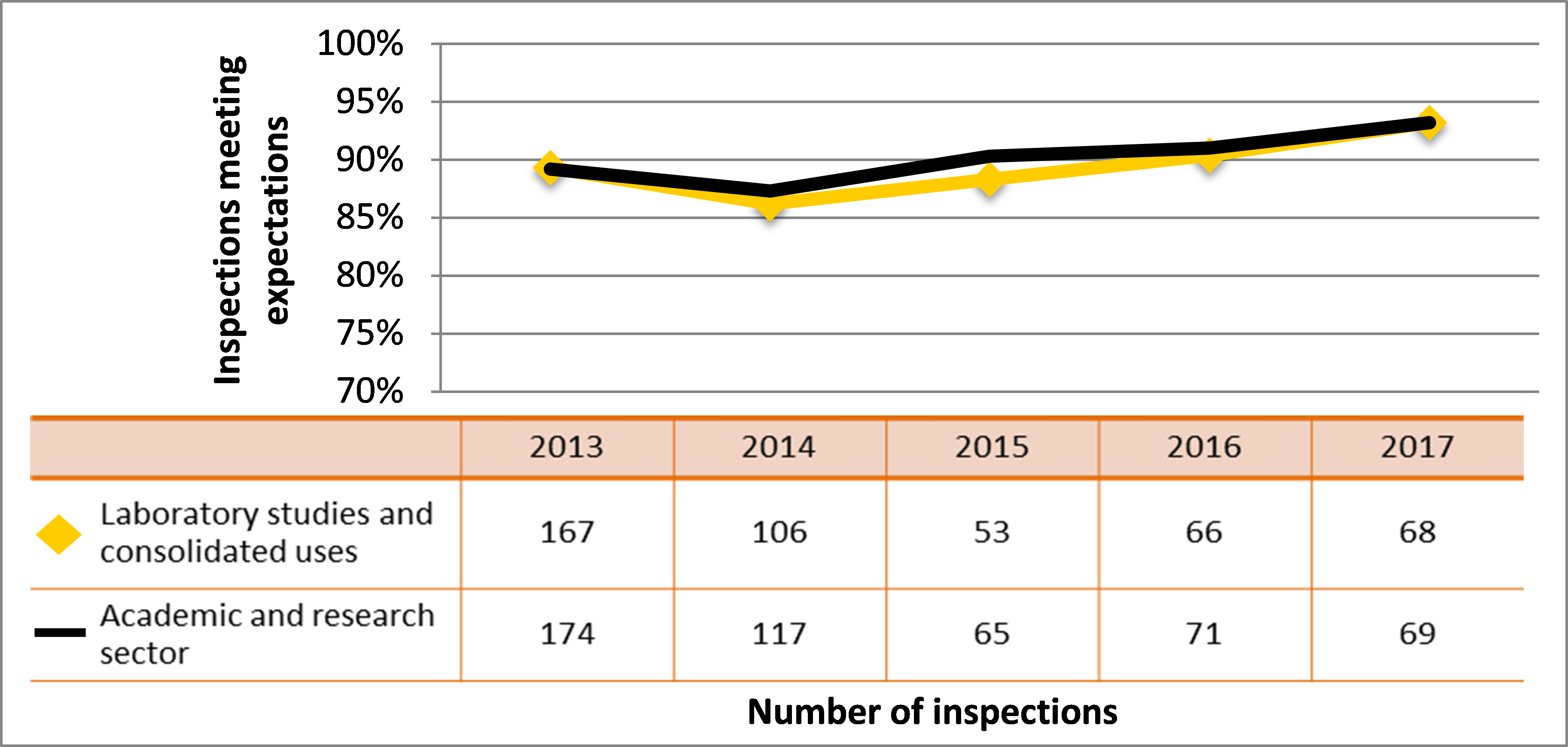
Description
| Sector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Laboratory studies and consolidated uses | 167 | 106 | 53 | 66 | 68 |
| Academic and research sector | 174 | 117 | 65 | 71 | 69 |
Note: The number of inspections shown in the academic and research row is the aggregate for the entire sector, including subsectors not highlighted in this report.
8.3.5 Security
The overall compliance rating for the security SCA for licensees in the academic and research sector was 96% (66 of 69 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 47.
Figure 47: Academic and research sector performance – details of inspection ratings for security, 2014–17
Description
| Inspection ratings | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| Fully satisfactory or satisfactory | 120 | 64 | 70 | 66 |
| Below expectations | 3 | 6 | 3 | 3 |
| Unacceptable | 0 | 0 | 0 | 0 |
9 Commercial sector
The commercial sector encompasses a number of licensed activities related to the production, processing, storage and distribution of nuclear substances, the calibration of radiation detection instruments, as well as the servicing of radiation devices and Class II prescribed equipment as a commercial enterprise. In 2017, this sector accounted for 246 CNSC licences and a total of 2,666 workers, 1,734 of whom were designated as nuclear energy workers (NEWs).
The results of CNSC staff’s evaluation of the regulatory performance of all commercial sector licensees are included in the overall results. The following five subsectors are highlighted in further detail:
- isotope production accelerators – medium-risk activity
- processing of nuclear substances – medium-risk activity
- distribution of nuclear substances – medium-risk activity
- servicing of radiation devices and prescribed equipment – medium-risk activity
- calibration of radiation devices and prescribed equipment – medium-risk activity
9.1 Sector overview
Figure 48: Installation of a fixed gauge by a servicing company (Source: CNSC)

The commercial sector encompasses a number of licensed activities related to the production, processing, storage and distribution of nuclear substances, and the calibration and servicing of radiation devices for commercial gain.
Isotope-production cyclotrons can produce a range of different radioisotopes widely used in the diagnosis, management and treatment of disease. Most licensees in the processing of nuclear substances subsector process isotopes to provide products and services used for the prevention, diagnosis and treatment of disease.
Distributors of radiation devices and nuclear substances are the link between the manufacturer and the end user. In some cases (for example, smoke detectors), end users are not required to hold licences for devices; however, companies that distribute such products in Canada are.
A licence is required to possess equipment for calibrating radiation detection instruments such as radiation survey meters. These licensees use nuclear substances and radiation devices to determine the response of radiation detection instruments.
Installation, repair and non-routine maintenance of radiation devices and prescribed equipment located in Canada requires a servicing licence issued by the Canadian Nuclear Safety Commission (CNSC), even if the licensee’s headquarters is located outside Canada.
9.2 Summary of safety assessment
The commercial sector continued to show good safety performance in 2017.
Doses received by NEWs in this sector remained low, with the majority of workers receiving doses below 1 mSv (millisievert).
Of all the licensees inspected in 2017, the majority were found to be compliant in the four safety and control areas (SCAs) covered in this report:
- 93% were compliant in management system
- 94% were compliant in operating performance
- 95% were compliant in radiation protection
- 94% were compliant in security
In cases where non-compliances were noted, licensees took appropriate corrective actions, satisfactory to CNSC staff, to address the non-compliances.
Results are presented for the commercial sector as a whole and also for the subsectors. However, discerning trends in the subsectors is difficult due to the small number of inspections conducted on licensees in each subsector.
In 2017, the CNSC issued an AMP to an individual who was transporting passengers while also transporting nuclear substances, which is prohibited under the regulations. Since the individual conducted that activity in 2016, details are not presented in this year’s report. The circumstances surrounding this incident were presented to the Commission in December 2016.
Additional details about the enforcement actions are found in appendix B.
9.3 Safety performance measures
9.3.1 Doses to workers
NEWs in the isotope production accelerators and processing of nuclear substances subsectors continued to receive higher doses than workers in other commercial subsectors, as shown in figure 49. This is due to their manual handling of nuclear substances and activated cyclotron components. In 2017, more than 97% of NEWs in these two subsectors received doses below 5 mSv.
Annual effective doses for NEWs in the isotope production accelerators subsector from 2013 to 2017 are shown in figure 50. Annual effective doses for NEWs in the processing of nuclear substances subsector from 2013 to 2017 are shown in figure 51.
Figure 49: Commercial sector performance comparison with select subsectors – annual effective doses to NEWs in 2017
Description
| Sector | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| Isotope production | 76 | 13 | 12 | – | – | – |
| Processing of nuclear substances | 160 | 44 | 86 | 9 | – | – |
| Distribution | 241 | 7 | 11 | 4 | – | – |
| Servicing | 766 | 35 | 40 | 6 | – | – |
| Calibration | 137 | 6 | 10 | – | – | – |
| Commercial sector | 1,437 | 119 | 159 | 19 | – | – |
Note: The total number of NEWs shown in the commercial sector is the aggregate for the entire sector, including subsectors not highlighted in this report.
Figure 50: Isotope production accelerators subsector performance – annual effective doses to NEWs, 2013‒17
Description
| Year | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv |
|---|---|---|---|---|---|---|
| 2013 | 101 | 20 | 42 | 6 | – | – |
| 2014 | 88 | 15 | 45 | 9 | – | – |
| 2015 | 123 | 18 | 47 | 14 | – | – |
| 2016 | 68 | 12 | 23 | 2 | – | – |
| 2017 | 76 | 13 | 12 | – | – | – |
Figure 51: Processing of nuclear substance subsector performance – annual effective doses to NEWs, 2013–17
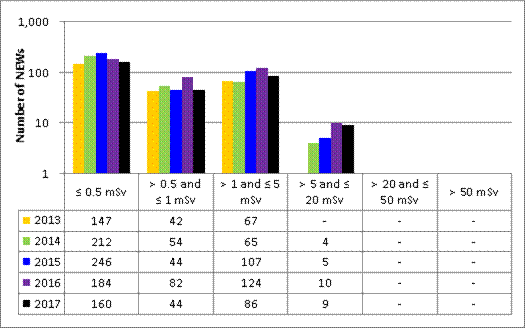
Description
| Year | ≤ 0.5 | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | >50 mSv |
|---|---|---|---|---|---|---|
| 2013 | 147 | 42 | 67 | – | – | – |
| 2014 | 212 | 54 | 65 | 4 | – | – |
| 2015 | 246 | 44 | 107 | 5 | – | – |
| 2016 | 184 | 82 | 124 | 10 | – | – |
| 2017 | 160 | 44 | 86 | 9 | – | – |
9.3.2 Management system
The overall compliance rating for management system in the commercial sector was 93% (53 of 58 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 52. A sector-to-subsector comparison of inspection ratings is provided in figure 53.
Figure 52: Commercial sector performance – details of inspection ratings for management system, 2015‒17
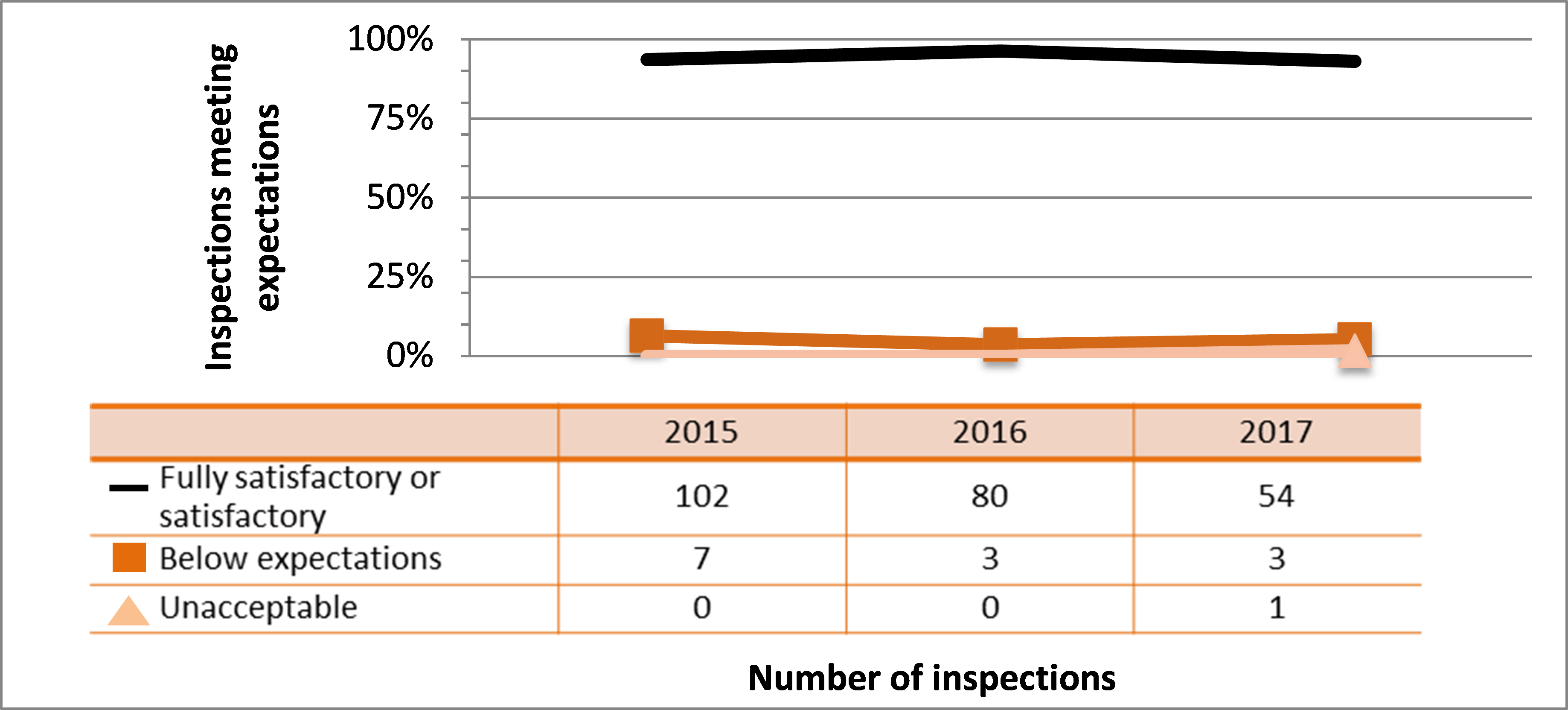
Description
| Inspection ratings | 2015 | 2016 | 2017 |
|---|---|---|---|
| Fully satisfactory or satisfactory | 102 | 80 | 54 |
| Below expectations | 7 | 3 | 3 |
| Unacceptable | 0 | 0 | 1 |
Figure 53: Commercial sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for management system, 2015–17
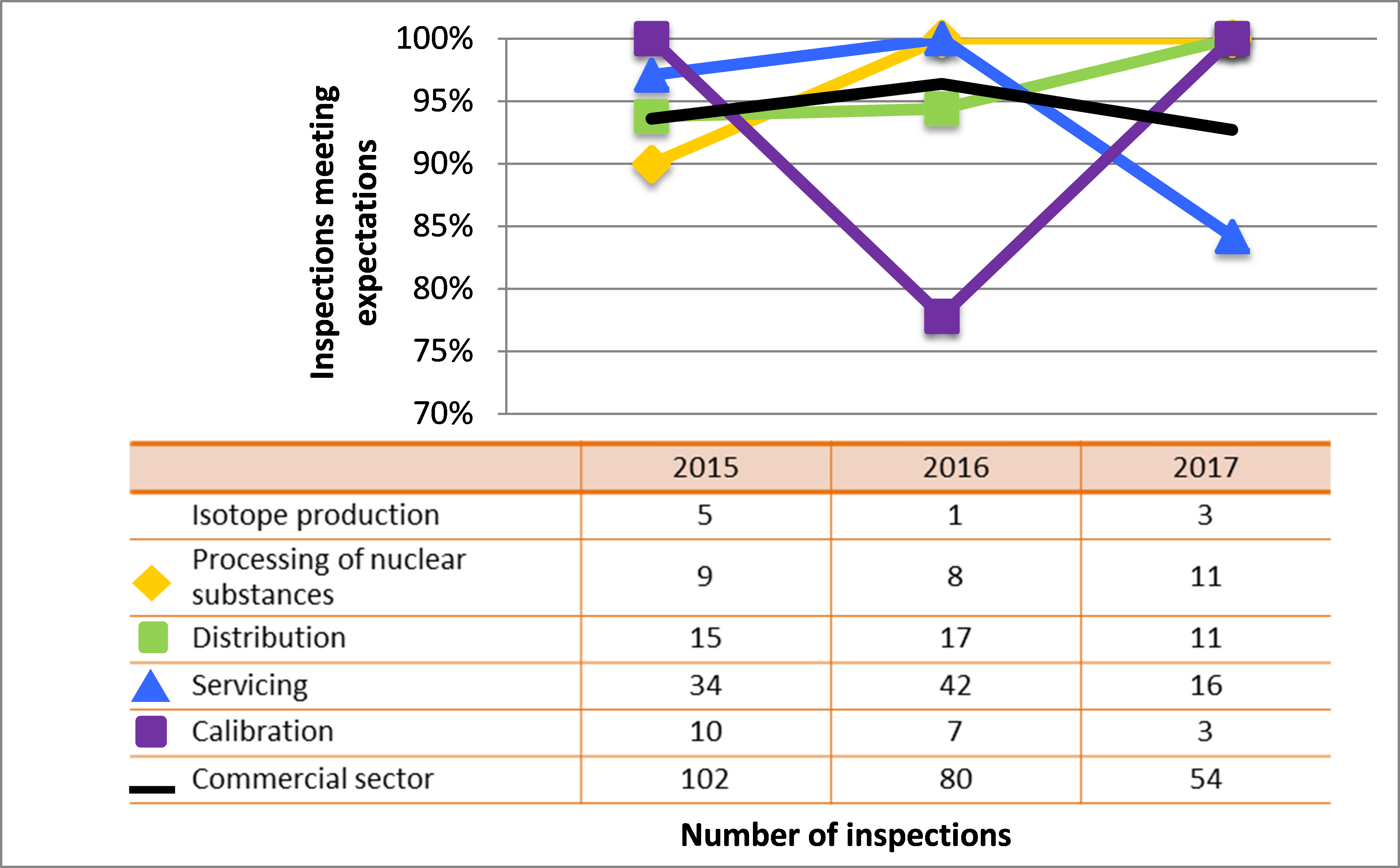
Description
| Commercial subsector | 2015 | 2016 | 2017 |
|---|---|---|---|
| Isotope production | 5 | 1 | 3 |
| Processing of nuclear substances | 9 | 8 | 11 |
| Distribution | 15 | 17 | 11 |
| Servicing | 34 | 42 | 16 |
| Calibration | 10 | 7 | 3 |
| Commercial sector | 102 | 80 | 54 |
Note: The number of inspections shown in the commercial sector row is the aggregate for the entire sector, including subsectors not highlighted in this report. The trend line was not provided for the isotope production accelerators subsector due to the low number of inspections conducted.
9.3.3 Operating performance
The overall compliance rating for operating performance in the commercial sector was 94% (59 of 63 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 54. This is consistent with the performance over the last five years. A sector-to-subsector comparison for operating performance ratings is provided in figure 55.
The most common non-compliances in this SCA were workers’ failure to follow licensee procedures or use the provided safety equipment. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 54: Commercial sector performance – details of inspection ratings for operating performance, 2013–17
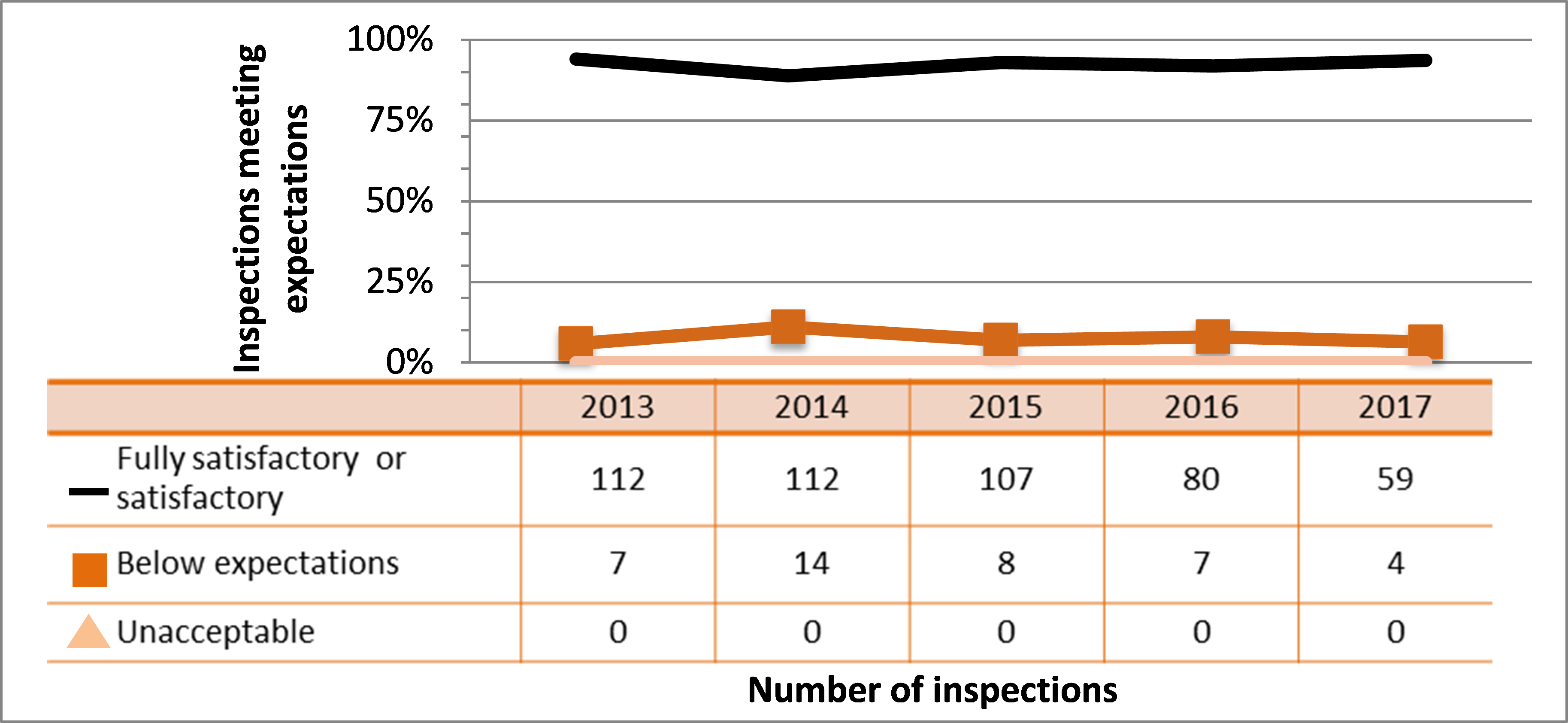
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 112 | 112 | 107 | 80 | 59 |
| Below expectations | 7 | 14 | 8 | 7 | 4 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
Figure 55: Commercial sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for operating performance, 2013‒17
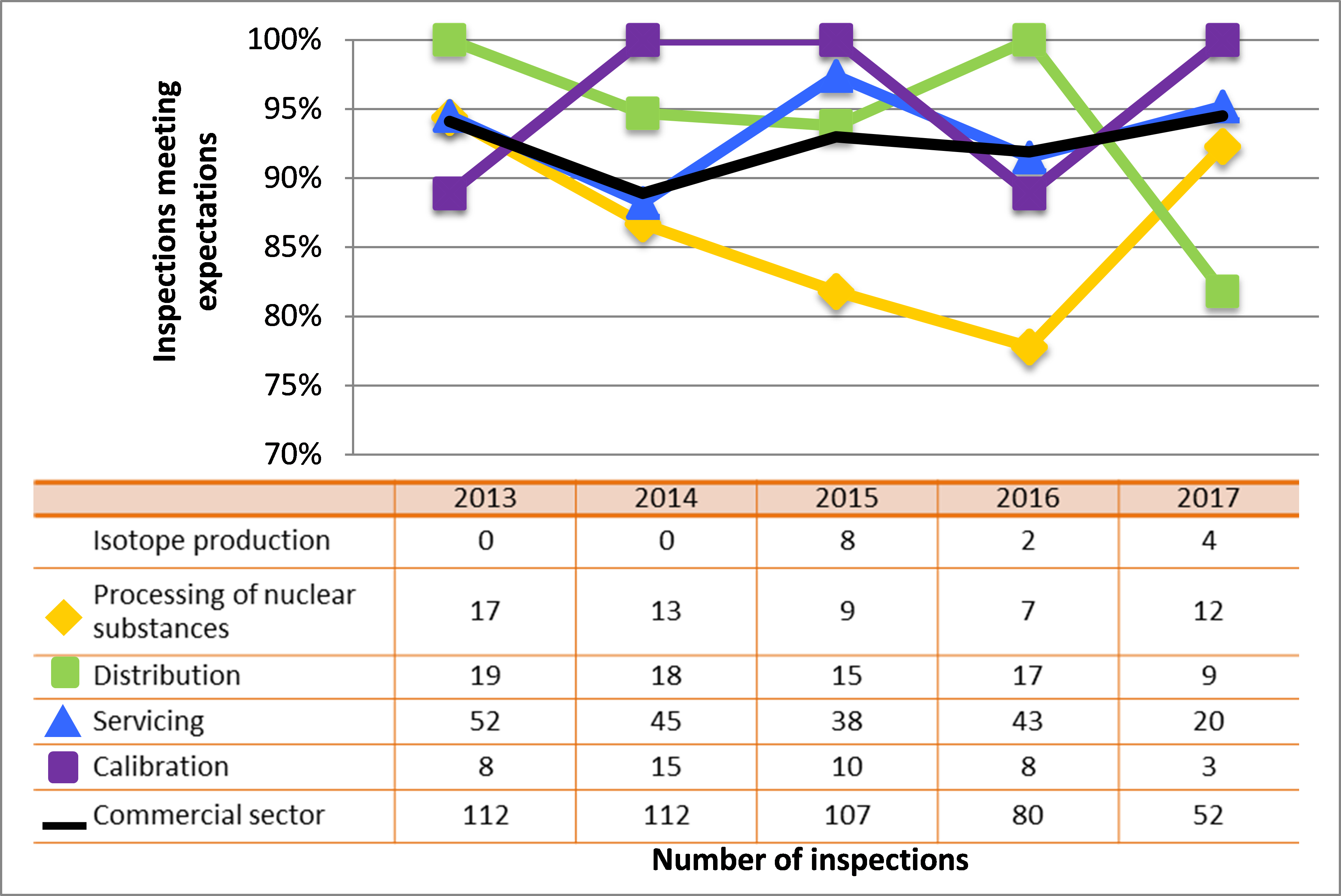
Description
| Commercial subsector | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Isotope production | 0 | 0 | 8 | 2 | 4 |
| Processing of nuclear substances | 17 | 13 | 9 | 7 | 12 |
| Distribution | 19 | 18 | 15 | 17 | 9 |
| Servicing | 52 | 45 | 38 | 43 | 20 |
| Calibration | 8 | 15 | 10 | 8 | 3 |
| Commercial sector | 112 | 112 | 107 | 80 | 52 |
Note: The number of inspections shown in the commercial sector row is the aggregate for the entire sector, including subsectors not highlighted in this report. The trend line was not provided for the isotope production accelerators subsector due to the low number of inspections conducted.
9.3.4 Radiation protection
The overall compliance rating for radiation protection in the commercial sector was 94% (59 of 62 inspections were fully satisfactory or satisfactory) in 2017, as shown in figure 56. This is an improvement in performance, and the highest rate of fully satisfactory and satisfactory inspections in the past five years. A sector-to-subsector comparison for radiation protection ratings is provided in figure 57.
The most common non-compliance was licensees’ failure to implement radiation protection programs that keep doses to workers and the public ALARA. CNSC staff tracked all items of non-compliance until the licensee addressed them in a manner that was satisfactory to the CNSC.
Figure 56: Commercial sector performance – inspection ratings for radiation protection, 2013–17
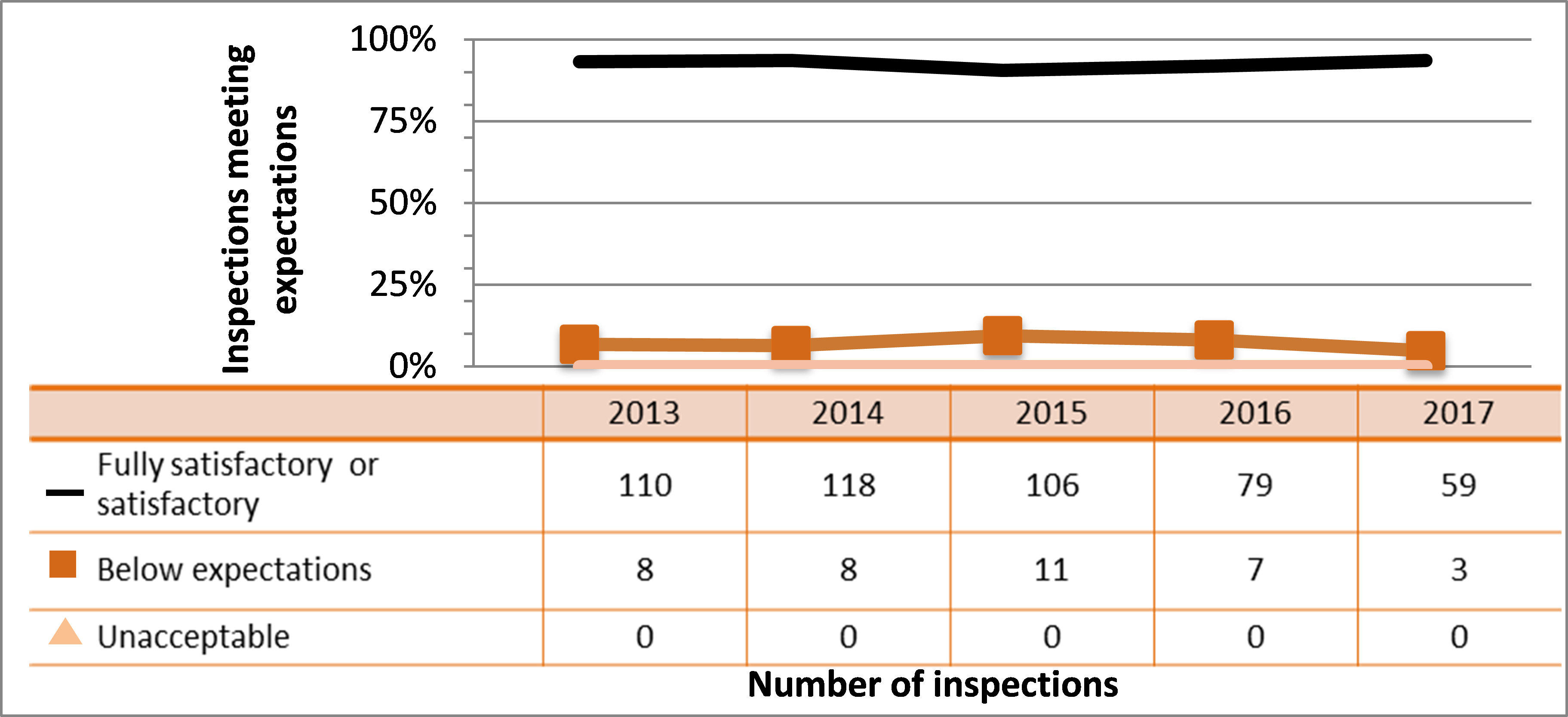
Description
| Inspection ratings | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 110 | 118 | 106 | 79 | 59 |
| Below expectations | 8 | 8 | 11 | 7 | 3 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
Figure 57: Commercial sector performance comparison with highlighted subsectors – inspection ratings meeting or exceeding expectations for radiation protection, 2013–17
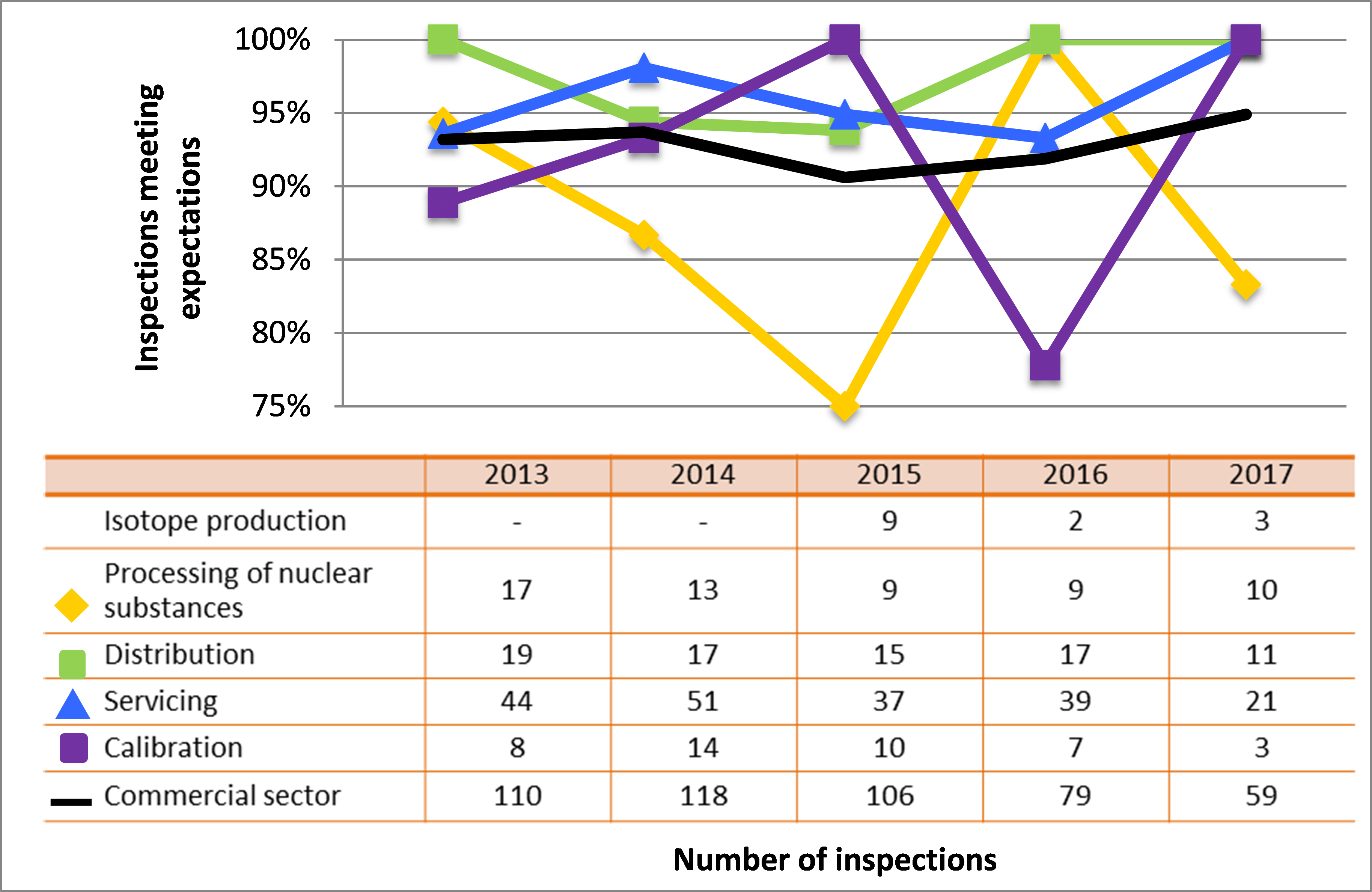
Description
| Commercial subsectors | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| Isotope production | – | – | 9 | 2 | 3 |
| Processing of nuclear substances | 17 | 13 | 9 | 9 | 10 |
| Distribution | 19 | 17 | 15 | 17 | 11 |
| Servicing | 44 | 51 | 37 | 39 | 21 |
| Calibration | 8 | 14 | 10 | 7 | 3 |
| Commercial sector | 110 | 118 | 106 | 79 | 59 |
Note: The number of inspections shown in the commercial sector row is the aggregate for the entire sector, including subsectors not highlighted in this report. The trend line was not provided for the isotope production accelerators subsector due to the low number of inspections conducted.
9.3.5 Security
The overall compliance rating for security in the commercial sector dropped to 93% (46 of 49 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in figure 58 figure 58. Previously the level was between 97 and 99%.
Figure 58: Commercial sector performance – details of inspection ratings for security, 2014–17
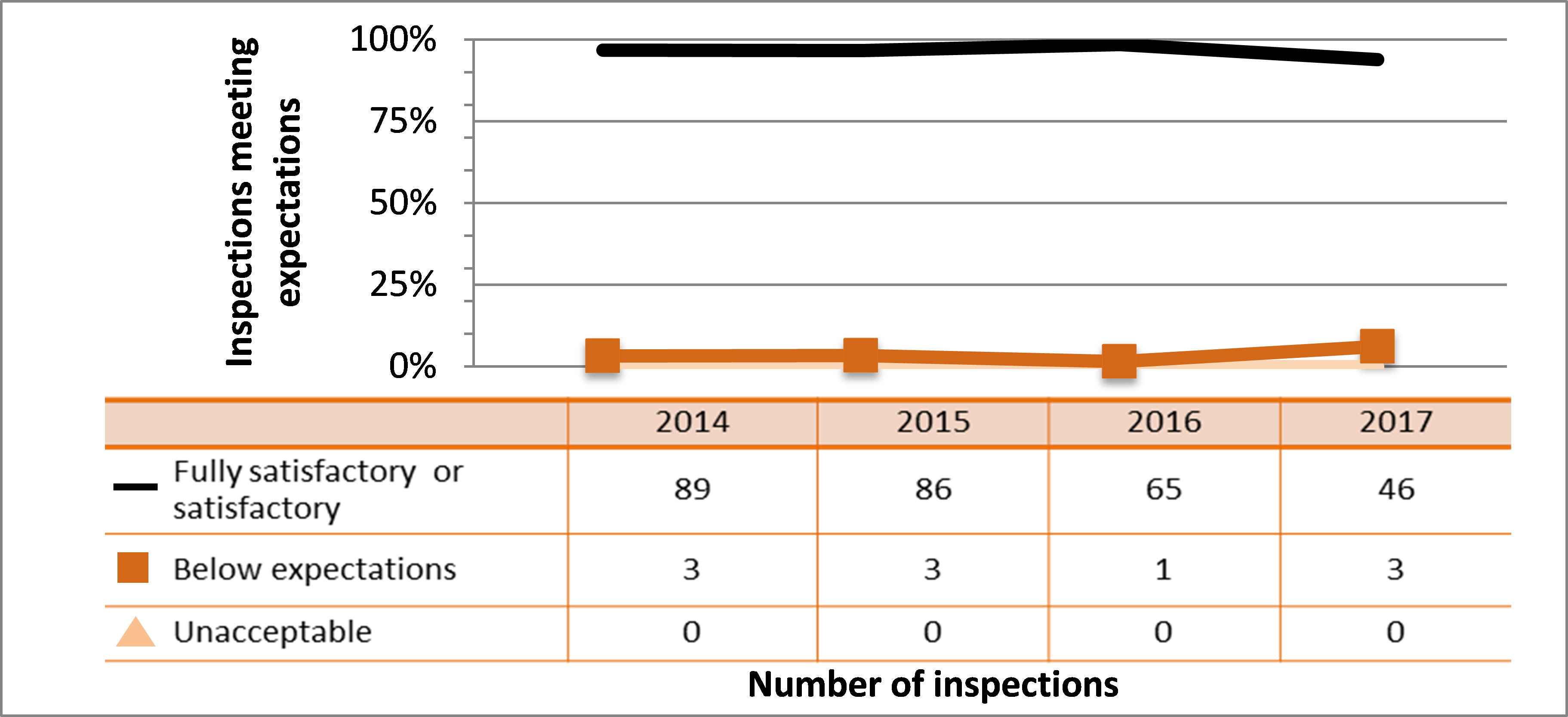
Description
| Inspection ratings | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| Fully satisfactory or satisfactory | 89 | 86 | 65 | 46 |
| Below expectations | 3 | 3 | 1 | 3 |
| Unacceptable | 0 | 0 | 0 | 0 |
10 Waste nuclear substance sector
The waste nuclear substance sector encompasses licensed activities related to the safe management of nuclear substances that are considered to be radioactive waste as described in CNSC regulatory policy P-290, Managing Radioactive Waste.
In 2017, there were six licences in this sector and 137 designated nuclear energy workers (NEWs).
Figure 59: Inspection of low-level waste storage area for nuclear substances (Source: CNSC)

10.1 Sector overview
Licensees in the waste nuclear substance sector are authorized by the designated officer to manage, handle, store and process low-level radioactive waste generated from licensed nuclear facilities and activities. The types of waste handled include low-level waste from research laboratories (e.g., gloves, paper towels, liquid scintillation vials), as well as slightly contaminated metals, laundry, tooling and equipment from other types of nuclear facilities (e.g., nuclear power plants and fuel cycle facilities). The waste is temporarily stored, sorted, decontaminated or repackaged before being either returned to the facility or sent to licensed waste management facilities. One licensee in this sector is involved solely in the activity of transporting potentially contaminated laundry from Canadian nuclear power plants to cleaning facilities.
The Canadian Nuclear Safety Commission (CNSC) requires that waste licensees maintain an acceptable environmental protection program for any licensed activity that can involve a potential release to the environment. The licensee environmental protection program is in place to manage and monitor any environmental emissions from the activity. Licensees are required to report on environmental releases to the CNSC. Due to the low-risk nature of the licensed activities in the waste nuclear substance sector, emissions have historically been below levels that would pose a risk to the public or the environment. CNSC staff are satisfied that there are adequate measures and programs in place to protect the public and the environment.
10.2 Summary of safety assessment
The waste nuclear substance sector continued to show satisfactory safety performance in 2017.
Figure 60: Licensee storing waste nuclear substances (Source: CNSC)

Doses received by NEWs in this sector remained very low, with all workers receiving doses below 1 mSv.
All licensees received satisfactory ratings in the five SCAs covered in this report: management system, operating performance, radiation protection, security and environmental protection. Overall, the licensees inspected were found to be in compliance with the inspection criteria. CNSC staff ensured that licensees for which non-compliances were identified took appropriate corrective actions to address the non-compliances. Any non-compliances found during inspections do not pose an immediate or unreasonable risk to the health and safety of persons or to the environment.
No enforcement actions were issued to licensees in this sector in 2017.
10.3 Safety performance measures
10.3.1 Doses to workers
Doses to NEWs in the waste nuclear substance sector continue to be low. All worker doses were below 1 mSv in 2017, with the majority of doses being below 0.5 mSv (table 5).
| ≤ 0.5 mSv | > 0.5 and ≤ 1 mSv | > 1 and ≤ 5 mSv | > 5 and ≤ 20 mSv | > 20 and ≤ 50 mSv | > 50 mSv | |
|---|---|---|---|---|---|---|
| 2013 | 390 | 0 | 0 | 0 | 0 | 0 |
| 2014 | 210 | 1 | 0 | 0 | 0 | 0 |
| 2015 | 144 | 5 | 1 | 0 | 0 | 0 |
| 2016 | 89 | 1 | 0 | 0 | 0 | 0 |
| 2017 | 132 | 5 | 0 | 0 | 0 | 0 |
10.3.2 Management system
The overall compliance rating for management system in the waste nuclear substance sector was 100% (4 out of 4 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in table 6. No licensees received below expectations or unacceptable ratings in this SCA. The licensees continue to maintain the processes, programs and resources required to ensure that the licensee achieves its safety objectives, continuously monitors its performance against those objectives, and fosters a healthy safety culture.
| 2015 | 2016 | 2017 | |
|---|---|---|---|
| Fully satisfactory or satisfactory | 8 | 4 | 4 |
| Below expectations | 0 | 0 | 0 |
| Unacceptable | 0 | 0 | 0 |
10.3.3 Operating performance
The overall compliance rating for operating performance in the waste nuclear substance sector was 100% (4 out of 4 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in table 7. No licensees received below expectations or unacceptable ratings in this SCA. The licensees continue to provide workers with appropriate procedures for the safe use of nuclear substances and prescribed equipment, maintain records that demonstrate compliance, and ensure that workers follow procedures.
| 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 6 | 9 | 8 | 4 | 4 |
| Below expectations | 0 | 0 | 0 | 0 | 0 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
10.3.4 Radiation protection
The overall compliance rating for radiation protection in the waste nuclear substance sector was 100% (4 out of 4 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in table 8. No licensees received unacceptable ratings in this SCA. The licensees continue to monitor worker doses; maintain oversight of operational activities; and institute effective workplace practices that emphasize the use of time, distance and shielding to minimize exposure to radiation, and emphasize the use of appropriate protective equipment.
| 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|
| Fully satisfactory or satisfactory | 6 | 9 | 8 | 4 | 4 |
| Below expectations | 0 | 0 | 0 | 0 | 0 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
10.3.5 Environmental protection
The overall compliance rating for environmental protection in the waste nuclear substance sector was 100% (4 out of 4 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in table 9. No licensees received below expectations or unacceptable ratings in this SCA. The licensees continue to manage and monitor environmental releases as a result of licensed activities. These releases are kept well below regulatory limits. There were no unplanned releases to the environment as a result of licensed activities.
| 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|
| Fully satisfactory or Satisfactory | 6 | 9 | 8 | 4 | 4 |
| Below expectations | 0 | 0 | 0 | 0 | 0 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
10.3.6 Security
The overall compliance rating for security in the waste nuclear substance sector was 100% (4 out of 4 inspections were rated fully satisfactory or satisfactory) in 2017, as shown in table 10. No licensees received unacceptable ratings in this SCA. The licensees continue to maintain an effective security program to prevent the loss, illegal use, illegal possession or illegal removal of nuclear substances, prescribed equipment and prescribed information.
Table 10 : Waste nuclear substance sector performance – details of inspection ratings for security, 2013–17
| 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|
| Fully satisfactory or Satisfactory | 6 | 9 | 8 | 4 | 4 |
| Below expectations | 0 | 0 | 0 | 0 | 0 |
| Unacceptable | 0 | 0 | 0 | 0 | 0 |
11 Conclusion
Canadian Nuclear Safety Commission (CNSC) staff continued their ongoing regulatory oversight of licensees in the medical, industrial, academic and research, commercial, and waste nuclear substance sectors. Staff conducted compliance verification activities consisting of field inspections, desktop reviews and technical assessments of licensee activities, and concluded that the use of nuclear substances in Canada is safe. The evaluations of findings for the safety and control areas (SCAs) covered in this report show that, overall, licensees made adequate provisions for the protection of the health, safety and security of persons and the environment from the use of nuclear substances, and took the measures required to implement Canada’s international obligations.
Figure 61: Inspection of brachytherapy equipment in a hospital (Source: CNSC)

Compliance verification
In 2017, CNSC staff conducted 944 inspections to verify compliance with CNSC regulatory requirements across all sectors, including 160 security inspections related to the first phase of the implementation of REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources. Of inspected licensees, the majority were found to be compliant in the SCAs covered in this report:
- 97% were compliant in management system
- 85% were compliant in operating performance
- 85% were compliant in radiation protection
- 90% were compliant in security
- 100% of waste nuclear substances licensees received satisfactory ratings for environmental protection
Licensees that failed to meet requirements took appropriate corrective measures to address non-compliances found during inspections. CNSC staff systematically tracked all non-compliances until licensees took the appropriate corrective measures to address them. All corrective measures put in place by licensees were reviewed by CNSC staff and found to be satisfactory.
Doses to workers
Doses to workers remained very low in 2017, consistent with previous years. One NEW received an equivalent dose above the CNSC regulatory dose limit of 500 millisieverts (mSv) for the skin of the hands.
Enforcement actions
In 2017, the CNSC took escalated enforcement actions in 24 instances. It issued 18 orders and 6 administrative monetary penalties (AMPs) to ensure that the health and safety of workers, the Canadian public and the environment were being adequately protected. Most of the enforcement actions were taken against licensees in the industrial sector, consistent with trends from previous years. All licensees to whom orders were issued have implemented corrective measures, which were reviewed by CNSC staff and found to be satisfactory. In one case in which the licence was revoked, the order remains open. Five of the six AMPs issued in 2017 have been paid.
Reported events
Licensees reported 146 events to the CNSC that are covered in this report, all of which were assessed by CNSC staff. Of the total number of events reported, 144 were categorized as level 0 (no safety significance) on the International Nuclear and Radiological Event Scale. One event was ranked as level 1 (anomaly) due to the quantity of nuclear substances involved and the type of event reported. The remaining event – ranked at level 2 (incident) – resulted in a NEW receiving a dose to the skin of the hands above the regulatory limits.
There were no releases of nuclear substances to the environment that had an adverse radiological impact or that resulted in a person receiving a dose in excess of the regulatory limit for members of the public.
Regulatory focus in 2018
The CNSC’s focus in 2018 continues to be on effective regulatory oversight and continuous improvement. Activities that will be undertaken in 2018 include:
- verifying the implementation of the requirements in REGDOC‑2.12.3, Security of Nuclear Substances: Sealed Sources, which came into force on May 31, 2018 for Category 3, 4 and 5 sealed sources
- rolling out an information program targeting portable gauge users, including an updated user booklet and a safety video that have been developed to address the trends of decreasing compliance and relatively high number of events, relative to other subsectors
- continuing the implementation of the strategy approved by the Commission for reviewing the success factors of radiation safety officers and radiation protection programs
- reviewing internal processes and procedures to ensure that they are agile and sufficient to effectively regulate new technologies, new applications of existing technologies, and new types of prescribed equipment
- developing the first revision of CSA Group document CSA PCP-09, Certified Exposure Device Operator Personnel Certification Guide
-
finalizing the following regulatory documents, which were posted for public comment in 2017 and are expected
to be published in 2018:
- REGDOC-1.4.1, Licence Application Guide: Class II Nuclear Facilities and Prescribed Equipment
- REGDOC-1.5.1, Licence Application Guide: Certification of Radiation Devices or Class II Prescribed Equipment
- REGDOC-2.1.2, Safety Culture
- REGDOC-2.5.5, Design of Radiography Installations
- REGDOC-2.7.3, Radiation Protection Guidelines for Safe Handling of Decedents
Conclusion
The use of nuclear substances in Canada is safe. Adequate provisions for the protection of the health, safety, security, and the environment from the use of nuclear substances are in place.
Figure 62: Portable gauge being used at a construction site (Source: CNSC)

Appendix A: Radiation exposure
Non-occupational exposure to radiation can occur in many situations. For example, a person may be exposed to radiation during an airplane flight or by undergoing a medical procedure such as a chest X-ray. Natural background radiation contributes to radiation exposure received by all persons living on earth. The average annual dose from natural background radiation is approximately 1.8 mSv (millisieverts) in Canada and 2.4 mSv worldwide. Among major Canadian cities, Winnipeg has the highest annual average dose from background radiation at 4.1 mSv.
Figure 63 provides some perspective on these situations as they relate to doses to the public as well as occupational radiation exposures received by workers as a result of both nuclear activities licensed by the Canadian Nuclear Safety Commission (CNSC) and natural sources of radiation.
Figure 63: Doses in perspective
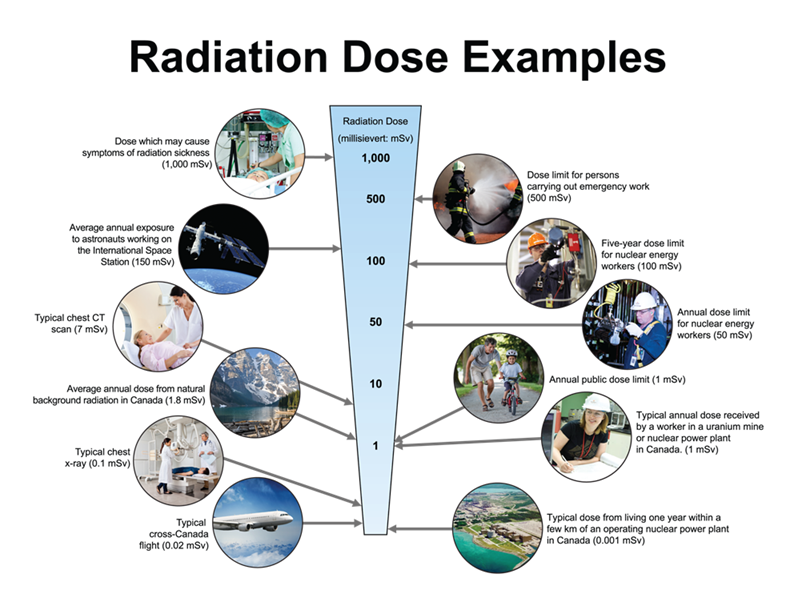
Description
Doses in perspective - Description: Image shows a scale running from 0 to 1,000 mSv and the various situations at each point in the scale where a person may be expected to receive that dose. A typical dose received from living one year within a few km of an operating nuclear plant in Canada is 0.001 mSv. A typical dose received from a cross-Canada flight is 0.02 mSv. A typical dose from a chest x-ray is 0.1 mSv. A typical annual dose received by a worker in a uranium mine or nuclear power plant in Canada is 1 mSv. The annual public dose limit is also 1 mSv. The average annual dose from natural background radiation in Canada is 1.8 mSv. A typical dose received from a chest CT scan is 7 mSv. The annual dose limit for nuclear energy workers is 50 mSv. The five-year dose limit for nuclear energy workers is 100 mSv. The average annual exposure to astronauts working on the International Space Station is 150 mSv. The dose limit for persons carrying out emergency work is 500 mSv. The dose which may cause symptoms of radiation sickness is 1,000 mSv.
Ascertaining effective dose
In this report, effective dose refers to the dose received by the whole body. Each licensee is required to ascertain the effective dose received by each worker engaged in activities authorized under their CNSC licence. Doses may be ascertained by direct measurement (through monitoring) or by estimation, in accordance with the Radiation Protection Regulations. The Radiation Protection Regulations also stipulate that the licensee must use a licensed dosimetry service for monitoring every nuclear energy worker (NEW) who has a reasonable probability of receiving an effective dose of greater than 5 mSv per year. However, regardless of the potential for occupational exposure, licensees conducting licensed activities in certain industries, such as industrial radiography, are always required to use a licensed dosimetry service provider to ascertain doses for the NEWs they employ (under subsection 30(3) of the Nuclear Substances and Radiation Devices Regulations).
When a dose limit is exceeded
In a situation where a worker may have exceeded a regulatory dose limit, licensees are required to remove the worker from any activities that may add to his or her dose, investigate the cause of the exposure, take action to prevent a recurrence, and report to the CNSC. CNSC staff review the information provided by the licensee following each investigation. Depending on the circumstances, the Commission, or in most cases a designated officer authorized by the Commission, may authorize the worker to return to work according to the process defined in the Radiation Protection Regulations . The return-to-work authorization may specify conditions and prorated dose limits for the remainder of the dosimetry period.
Appendix B: Enforcement actions issued in 2017
Canadian Nuclear Safety Commission (CNSC) inspectors and designated officers issued a total of 24 enforcement actions in the form of 18 orders and 6 administrative monetary penalties (AMPs) in 2017 to licensees covered by this report. Details of the orders issued are shown in table 11. Details of the AMPs are provided in table 12. Dates are in the year 2017 unless indicated otherwise.
| Issue date | Location | Licensee | Sector and subsector | Licensee response | Closure date |
|---|---|---|---|---|---|
| Jan.12 | St. Catharines, ON | Trenergy Inc. |
Industrial sector Industrial radiography |
Prohibited all workers not provided the required dosimetry from operating an exposure device. Retrained all workers in their obligations regarding the use and wearing of personal dosimetry. Submitted to the CNSC a remedial action plan, including changes to the radiation protection program. Corrected all items of non-compliance to the satisfaction of the CNSC. | Feb. 28 |
| Jan. 23 | Calgary, AB | Englobe Corp. |
Industrial sector Portable gauge |
Ceased using portable gauges at one base of operations until improvements were made to the implementation and oversight of the radiation protection program at that location and all items of non-compliance were corrected to the satisfaction of the CNSC. Corrected all items of non-compliance to the satisfaction of the CNSC. | Feb. 20 |
| Jan. 25 | Nackawic, NB | AV Groupe NB Inc. |
Industrial sector Fixed gauge |
Ceased all entries into vessels with radiation devices until the licensee changed their procedures for conducting vessel entries to ensure compliance with the CNSC’s requirements and until staff were trained on the new procedures. Corrected all items of non-compliance to the satisfaction of the CNSC. | Apr. 3 |
| Feb. 16 | Conception Bay South, NL | Newfoundland Recycling Ltd. |
Industrial sector X-ray analyzer |
Licensee took the device back into their possession and then transferred it to a company licensed to possess it. Once Newfoundland Recycling Ltd. was no longer in possession of the device, their licence was revoked. | Feb. 26, 2018 |
| May 15 | Longueil, QC | Labo S.M. Group International Inc. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Jun. 7 |
| May 18 | Laval, QC | Groupe ABS Inc. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Jun. 7 |
| May 24 | Calgary, AB | Sable Sands Solutions Inc. |
Industrial sector Fixed gauge |
Ceased operation and transport of radiation devices until changes were made to the implementation and oversight of the radiation protection program, and corrected all items of non-compliance to the satisfaction of the CNSC. | Jun. 20 |
| Jun. 17 | Laval, QC | Labo S.M. Group International Inc. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Jun. 29 |
| Aug. 17 | Montréal, QC | GHD Consultants Ltée. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Sep. 7 |
| Aug. 25 | Laval, QC | Englobe Corp. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Sep. 7 |
| Aug. 29 | Montréal, QC | SNC – Lavalin GEM Québec Inc. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Sep. 7 |
| Aug. 31 | Chicoutimi, QC | Inter-Cité Construction Ltd. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Sep. 7 |
| Sep. 11 | Whitehorse, YK | 42256 Yukon Inc. |
Industrial sector Portable gauge |
Ceased operation of portable gauges and placed them in secure storage until all non-compliances corrected to the satisfaction of the CNSC. | Sep. 22 |
| Sep. 13 | Brossard, QC | Groupe Conseil SCT Inc. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Nov. 1 |
| Sep. 18 | Mississauga, ON | GHD Consultants Ltée. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until the individual completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Oct. 26 |
| Oct. 2 | Penticton, BC | Seymour Pacific Developments Ltd. |
Industrial sector Portable gauge |
Ceased operation of portable gauges at this location until all non-compliances were corrected to the satisfaction of the CNSC. | Dec. 1 |
| Oct. 4 | Fort St. John, BC | Acciona Infrastructure Canada Inc. |
Industrial sector Portable gauge |
Removed one individual from work involving a portable gauge until they completed training on the licensee’s radiation protection program and matters related to the security of portable gauges, and demonstrated that they are working in accordance with CNSC regulations. Corrected all items of non-compliance to the satisfaction of the CNSC. | Dec. 31 |
| Oct. 23 | Whitecourt, AB | Bakos NDT Ltd. |
Industrial sector Industrial radiography |
Prevented one EDO from supervising trainees operating exposure devices until corrective measures were put in place and the licensee demonstrated to the satisfaction of the CNSC that it has effective control over the supervision of trainees. | Nov. 3 |
| Issue date | Licensee or individual | Sector and subsector | Reason for issuing AMP | Penalty amount | Closure date |
|---|---|---|---|---|---|
| Jan. 6 | Agnico-Eagle Mines Ltd. |
Industrial sector Fixed gauge |
Failure to conduct vessel entry according to the licence condition for vessel and hopper entry | $3,970 | Feb. 13 |
| Jan. 10 | R. Wessel |
Industrial sector Industrial radiography |
Failure to secure an exposure device to a vehicle | $300 | Feb. 14 |
| Feb. 24 | ArcelorMittal Canada Inc. |
Industrial sector Fixed gauge |
Failure to conduct vessel entry according to the licence condition for vessel and hopper entry | $3,970 | Mar. 6 |
| Mar. 10 | B. Ricignuolo |
Commercial sector Distribution |
Transporting passengers in a vehicle while transporting packages with II-YELLOW and III-YELLOW labels | $1,949 | Unpaid |
| Sep. 13 | Groupe ABS Inc. |
Industrial sector Portable gauge |
Multiple non-compliances including failure to maintain direct supervision of a portable gauge | $6,460 | Oct. 5 |
| Dec. 12 | B. Bakos |
Industrial sector Industrial radiography |
Failure of a certified EDO to directly supervise and continuously observe a trainee operating an exposure device | $1,270 | Dec. 15 |
Appendix C: List of reported events in 2017
Table 13 includes all reported events by licensees in 2017, categorized using the International Nuclear and Radiological Event Scale (INES) tool.
| # | Date | INES rating | Type | Sector | Event summary |
|---|---|---|---|---|---|
| 2954 | Jan. 4 | 0 | Breach of security | Academic and research | A door to an irradiator was left open and unlocked. The security of the source was not compromised, as this was one of three barriers protecting the source. Corrective actions were implemented to prevent recurrence. |
| 2951 | Jan. 5 | 0 | Missing or found | Academic and research | Two uranium pellets were found in a desk. The pellets predate the licensee’s inventory. They have been added to the licensee’s inventory. No contamination was detected. Dose rates were measured and indicated that exposure to individuals was unlikely. |
| 2952 | Jan. 6 | 0 | Malfunctioning device | Industrial | Two fixed gauges had the shutter stuck in the open position, their normal operating state. There was no additional risk to personnel, as the gauges are mounted away from traffic areas. |
| 2960 | Jan. 12 | 0 | Unplanned exposure | Industrial | A non-NEW received a dose below regulatory limits upon entering an area where industrial radiography was being performed. |
| 2956 | Jan. 17 | 0 | Damaged device | Industrial | An exposure device was dropped from a scaffold. There was minor damage to the front lip of the device. The device was removed from service and leak testing was performed. No leaks detected. |
| 2961 | Jan. 18 | 0 | Missing or found | Medical | An iodine-131 sealed source (brachyseed) used in cancer treatment was lost. This is a Category 5 source. The source was not recovered. |
| 3014 | Jan. 18 | 0 | Malfunctioning device | Industrial | The shutter on a portable gauge was stuck in the open position. |
| 2962 | Jan. 19 | 0 | Malfunctioning device | Industrial | The source in an exposure device would not retract to the shielded position. The device was removed from service and sent to the manufacturer for inspection. |
| 2963 | Jan. 23 | 0 | Malfunctioning device | Industrial | The gauge handle on a portable gauge was not functioning properly and the shutter was stuck in the open position. The device was removed from service until it was repaired. |
| 2985 | Jan. 25 | 0 | Damaged device | Industrial | An exposure device fell off a moving truck. The device was damaged but the source remained in the shielded position. No unusual dose rates were measured. The device was sent for repair. |
| 2967 | Jan. 26 | 0 | Damaged device | Industrial | A pin that limits movement of the handle on a portable gauge was missing, causing the source rod to retract beyond the safe position within the gauge housing. The worker verified there was no unusual dose rate. The gauge was removed from use until it was repaired. |
| 2968 | Jan. 31 | 0 | Malfunctioning device | Industrial | The shutter of a fixed gauge was not working properly. The device was repaired. |
| WNS1 | Feb. 3 | 0 | Fire | Waste nuclear substance | A fire occurred at a licensee site. The nuclear substances were safely stored outdoors and were unaffected by the fire. A memo to the Commission was provided on April 11, 2017. |
| 2975 | Feb. 3 | 0 | Packaging and transport | Medical | A package was mislabeled for transport. A full vial containing a medical isotope, technetium-99m, was shipped with a damaged seal. It spilled during transport. The spill was contained in the package. No contamination was detected on the outside of the package. |
| 2977 | Feb. 9 | 0 | Spill | Medical | A spill of a nuclear medicine isotope (technetium-99m) occurred in a nuclear medicine laboratory. The spill was cleaned up, but fixed contamination remained. This was left to decay, and the following day, no contamination was detected. |
| 2983 | Feb. 14 | 0 | Unplanned exposure | Industrial | A non-NEW received a dose below regulatory limits upon entering an area where industrial radiography was being performed. |
| 2990 | Feb. 17 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was struck by a vehicle at a construction site. No unusual dose rate was measured. The portable gauge was sent for repair. |
| 2998 | Feb. 20 | 0 | Missing or found | Medical | A Category 5 sealed source (cesium‑137) used for calibration was lost. The source was not recovered. |
| 2992 | Feb. 21 | 0 | Breach of security | Industrial | A break-in occurred at a portable gauge licensee’s location. This is the third break-in in two years. No portable gauges were stolen. The licensee upgraded their security. CNSC staff conducted a reactive security inspection. |
| 2996 | Feb. 21 | 0 | Packaging and transport | Industrial | A portable gauge was delivered to the wrong licensee by a courier. |
| 2999 | Feb. 23 | 0 | Packaging and transport | Industrial | A portable gauge was delivered to the wrong licensee by a courier. |
| 3005 | Mar. 1 | 2 | Unplanned exposure | Medical | A NEW in a nuclear medicine facility received skin contamination of the hands of 2,366 mSv (right hand) and 124 mSv (left hand). The contamination was transferred from a contaminated cart in the work area to the NEW’s hands. The worker did not notice the contamination on her hands until two days later. The worker has shown no negative effects. |
| 3003 | Mar. 3 | 0 | Damaged device | Industrial | An exposure device’s guide tube was damaged when a piece of metal fell on it. A source retrieval was successfully performed. The device was taken out of service. |
| 3034 | Mar. 3 | 0 | Missing or found | Academic and research | Two Category 5 sealed sources (cesium-137) were reported missing. They were recovered two weeks later. |
| 3006 | Mar. 7 | 0 | Unplanned exposure | Industrial | Workers (non-NEWs) removed a fixed gauge from operations with its shutter in the open position. No radiation survey was conducted before the device was removed. The gauge was stored with the shutter open for a month. The dose received by non-NEWs as a result of the event was below the regulatory limit. |
| 3015 | Mar. 7 | 0 | Contamination | Medical | A treadmill was contaminated as a result of a spill of technetium‑99m during a nuclear medicine procedure. The treadmill was not decontaminated after the incident and was used by three patients who were not receiving nuclear medicine procedures. The doses received were below regulatory limits. |
| 3008 | Mar. 8 | 0 | Unplanned exposure | Industrial | A non-NEW received a radiation dose below regulatory limits after entering an area where industrial radiography was being performed. |
| 3013 | Mar. 9 | 0 | Malfunctioning device | Industrial | A source disconnected from an exposure device while industrial radiography was being performed. The source was retrieved and returned to the shielded position. No unusual dose measurements were reported. The device was sent to the manufacturer for repair. |
| 3031 | Mar. 13 | 0 | Packaging and transport | Transport | A vehicle transporting nuclear medicine isotopes was involved in a motor vehicle collision. There was no damage to the packages. |
| 3022 | Mar. 16 | 0 | Spill | Commercial | A nuclear medicine isotope (iodine-131) spilled inside a dose calibrator in a hot cell. There was no thyroid update, no environmental releases and no overexposure as a result of this event. |
| 3024 | Mar. 16 | 0 | Malfunctioning device | Industrial | A fixed gauge had an elevated external dose rate when in the off position. Leak testing was conducted and no leaks were detected. The gauge remained in use with routine monitoring. |
| 3029 | Mar. 17 | 0 | Spill | Medical | A spill of fluorine-18 occurred behind shielding during sample preparation. No overexposures occurred as a result of this event. |
| 3020 | Mar. 20 | 0 | Malfunctioning device | Industrial | The shutter on a fixed gauge was stuck in the open position. A barrier was established until the device was removed. The device was repaired. |
| 3021 | Mar. 20 | 0 | Spill | Medical | A vial of a nuclear medicine isotope (technetium‑99m) broke and spilled inside a dose calibrator. The calibrator was removed from service. There was no overexposure as a result of this event. |
| 3023 | Mar. 21 | 0 | Packaging and transport | Medical | Two packages, each containing a technetium‑99m generator, were delivered to the wrong licensee. Each hospital received the other’s package. No possession limits were exceeded as a result of the incident. |
| WNS2 | Mar. 23 | 0 | Packaging and transport | Waste nuclear substance | A package received was misclassified as “unconditional release” and should have been classified as “excepted”. There was no impact on the health and safety of workers, the public or the environment as a result of this event. |
| 3032 | Mar. 27 | 0 | Spill | Commercial | A spill of a nuclear medicine isotope (technetium‑99m) occurred when a worker squeezed the vial too hard with a pair of tongs while trying to retrieve it after it fell. The spill was cleaned up. Shielding was added to cover the area with remaining contamination until it decayed. |
| 3028 | Mar. 28 | 0 | Damaged device | Industrial | The shutter on a fixed gauge was working incorrectly and not closing completely, resulting in radiation doses higher than normal. The portable gauge with an elevated radiation dose was transported in the incorrect package (Type A package used). The device was repaired. |
| 3033 | Mar. 29 | 0 | Missing or found | Industrial | A load of scrap metal triggered a portal monitor. The load was rejected and returned to Canada. The item that triggered the alarm contained 200 MBq of radium‑226. The item was transferred to a third party for disposal. |
| 3035 | Mar. 31 | 0 | Unplanned exposure | Industrial | A non-NEW received a radiation dose below regulatory limits after entering an area where industrial radiography was being performed. |
| 3057 | Apr. 6 | 0 | Packaging and transport | Commercial | A Type A package was damaged by water. The inner packaging was intact and there was no loss of containment and no contamination. |
| 3042 | Apr. 10 | 0 | Damaged device | Industrial | A portable gauge was damaged when it fell 7 m at a construction site. The source rod and body were intact. Leak testing was performed. No leaks were detected. The device was sent for servicing. |
| 3043 | Apr. 11 | 0 | Damaged device | Industrial | A worker broke the handle and the rod connecting it to the shutter when trying to remove a lock using a pry bar and hammer. The gauge was taken out of service until it was repaired. |
| 3044 | Apr. 12 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was hit by a large boulder at a construction site. The device was removed from service. Leak tests were performed. No leaks were detected. |
| 3062 | Apr. 19 | 0 | Packaging and transport | Industrial | Two Type A packages containing fixed gauges were delivered to the wrong location. |
| 3049 | Apr. 21 | 0 | Packaging and transport | Commercial | A package that had previously been used to transport nuclear medicine isotopes had contamination on the exterior above regulatory limits. |
| 3051 | Apr. 24 | 0 | Damaged device | Industrial | An exposure device was damaged after it fell 18 m from scaffolding. Radiation readings taken after the incident were within the normal range. The device was sent to the manufacturer for evaluation and repair. Leak tests were performed. No leaks were detected. |
| 3053 | Apr. 24 | 0 | Spill | Medical | There was a spill of a nuclear medicine isotope (technetium‑99m) in a laboratory. No regulatory doses were exceeded. |
| 3054 | Apr. 26 | 0 | Missing or found | Industrial | A portable gauge, in its Type A package, was left at the doorstep of a member of the public. In accordance with the Orphan Source Policy , CNSC issued a contract to a servicing licensee to pick up the gauge for disposal. |
| 3078 | Apr. 24 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. There was no damage to the portable gauge. Leak tests were performed. No leaks were detected. |
| 3056 | Apr. 29 | 0 | Spill | Medical | During a veterinary nuclear medicine procedure, there was a spill of iodine‑131. The veterinarian (a NEW) received a dose to the hand below regulatory limits. There was no contamination of the area. |
| 3058 | May 3 | 0 | Breach of security | Industrial | Workers left a fixed gauge unsecured and uninstalled at the end of their shift. The gauge was found 2.5 h later. It was installed later that night. The shutter was closed and locked; no overexposures occurred as a result. |
| 3059 | May 4 | 0 | Packaging and transport | Commercial | A licensee transported a sealed source in a Type A package; however, when they opened the package there was no source inside. The package was labelled incorrectly. It was later confirmed that the source had been shipped at an earlier date. |
| 3061 | May 7 | 0 | Missing or found | Industrial | Two sealed sources used for well-logging (Category 4 and Category 5) were lost during transport when they fell off the truck. The sources were recovered the same day. Leak tests were performed. No leaks were detected. |
| 3065 | May 11 | 0 | Spill | Commercial | A spill of gallium-68 occurred in a medical facility. There was no personnel contamination or release to the environment. The room was closed off until the isotope decayed to background levels. |
| 3067 | May 15 | 0 | Packaging and transport | Transport | The lead pig inside a Type A package was damaged. There was no indication that the package had been dropped. There was no contamination as a result of this event. |
| 3071 | May 15 | 0 | Damaged device | Industrial | A fixed gauge was damaged while it was being serviced. The shutter remained closed. The device was stored in a secure area until it was repaired. |
| 3068 | May 16 | 0 | Missing or found | Industrial | A portable gauge was lost during transit when it fell off the back of a truck. The gauge, still locked in its Type A package, was found three days later by a member of the public. |
| 3083 | May 18 | 0 | Breach of security | Industrial | A portable gauge was left unsecured and unattended at a construction site. |
| 3080 | Jun. 2 | 0 | Packaging and transport | Commercial | A package containing Mo-99 could not be delivered because it was improperly labelled. |
| 3081 | Jun. 5 | 0 | Damaged device | Industrial | A fixed gauge was damaged such that its shutter was stuck in the open position. The gauge was in a restricted access area, and a barrier was established until it could be dismounted. |
| 3084 | Jun. 7 | 0 | Spill | Academic and research | A vial containing technetium‑99m broke and spilled on the floor of a hot laboratory. The laboratory was closed and locked until it was decontaminated. There was no skin contamination as a result of the incident. |
| 3082 | Jun. 10 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by a truck at a construction site. The shutter remained closed. Leak tests were conducted. No leaks were detected. The device was sent to a service provider for disposal. |
| 3089 | Jun. 15 | 0 | Spill | Medical | A vial containing fluorine‑18 broke and spilled inside a shielded container. The spill occurred inside a hot laboratory. The lead container was put aside to decay. There was no overexposure as a result of this incident. |
| 3090 | Jun. 15 | 0 | Packaging and transport | Industrial | The Type A package used to transport a portable gauge was not locked during transport. There was no indication of tampering. |
| 3097 | Jun. 15 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. The Type A package was not damaged. Leak tests were conducted. No leaks were detected. |
| 3086 | Jun. 16 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by a truck at a construction site. The damaged gauge was packaged and sent for disposal. |
| 3088 | Jun. 20 | 0 | Missing or found | Academic and research | Five liquid scintillation counters with Category 5 sealed sources were reported missing. They have not been recovered. |
| 3087 | Jun. 21 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. Leak tests were performed on the gauge. No leaks were detected. |
| 3092 | Jun. 22 | 0 | Spill | Medical | A nuclear medicine NEW spilled technetium-99m on their forearms while dispensing a dose. There were no overexposures as a result of this event. |
| 3093 | Jun. 23 | 0 | Malfunctioning device | Industrial | The shutter of a fixed gauge was stuck in the open position. The device was removed and disposed of by a service provider. |
| 3101 | Jul. 6 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over at a construction site. The parts of the gauge were packaged and shipped to the manufacturer for leak testing and disposal. There were no leaks detected. |
| 3104 | Jul. 10 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. Neither the portable gauge nor its Type A package were damaged. Leak tests were conducted. No leaks were detected. |
| WNS3 | Jul. 13 | 0 | Flood | Waste nuclear substances | The water main supply to the facility prinkler system ruptured and caused flooding of the warehouse and processing areas. No radioactive materials were involved and no packages were damaged. The water did not enter the waste processing area and no waste was open in the area. Swipes and samples were taken; there was no contamination. |
| 3103 | Jul. 14 | 0 | Contamination | Academic and research | A nickel-63 Category 5 sealed source failed a leak test. The licensee isolated the source until it could be transferred for disposal. There was no loose contamination in the storage area where the source had been kept. |
| 3106 | Jul. 14 | 0 | Missing or found | Historical | A bin of scrap metal triggered a portal alarm at a salvage yard. The radioactive item, a historical cable coated with radioactive zinc sulfide, was removed from the bin by a consultant and disposed of. |
| 3107 | Jul. 14 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. Neither the portable gauge nor its Type A package was damaged. Leak tests were conducted. No leaks were detected. |
| 3108 | Jul. 18 | 0 | Malfunctioning device | Industrial | The shutter on a fixed gauge was stuck in the open position. Workers were able to close the shutter and lock the gauge, which was sent for disposal. |
| 3109 | Jul. 19 | 0 | Spill | Commercial | Iodine-131 spilled inside a shielded manufacturing box. The box was left to decay. There were no thyroid uptakes as a result of this event. There was no overexposure. |
| 3110 | Jul. 19 | 0 | Unplanned exposure | Industrial | A non-NEW received a radiation dose below regulatory limits as a result of an EDO not following procedures for erecting barriers while conducting industrial radiography. |
| 3069 | Jul. 20 | 0 | Malfunctioning device | Industrial | A source rod on a fixed gauge did not retract as it should. The tank where the gauge was located was closed until the gauge could be repaired. Dose rates around the tank were at normal levels. |
| 3111 | Jul. 25 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by a vehicle at a construction site. The source was in the shielded position. Leak tests were performed. No leaks were detected. The gauge was sent for disposal. The worker operating the portable gauge was injured as a result of the incident. |
| 3113 | Jul. 27 | 0 | Packaging and transport | Commercial | A vehicle transporting nuclear medicine substances was involved in a collision. Surveys taken at the time showed no indication of contamination. |
| 3114 | Jul. 27 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. Neither the Type A package nor the portable gauge was damaged. |
| 3115 | Jul. 27 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. Neither the Type A package nor the portable gauge was damaged. Leak tests were performed. No leaks were detected. |
| 3118 | Jul. 31 | 0 | Damaged device | Industrial | A portable gauge was damaged when it fell 1.2 m at an excavation site. Radiation surveys taken at the site were normal. The gauge was sent for repair. Leak tests were performed. No leaks were detected. |
| 3132 | Aug. 4 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was hit by a culvert that rolled off a stack. Leak tests were conducted. No leaks were detected. |
| 3119 | Aug. 8 | 0 | Malfunctioning device | Medical | Staff at a hospital were unable to exit a radiation treatment bunker due to problems with the door switch. The door was opened from the outside to allow the staff to exit. |
| 3170 | Aug. 8 | 0 | Breach of security | Industrial | A break-in occurred at a licensee’s fabrication shop. No nuclear substances were stolen. |
| 3121 | Aug. 11 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by a pick-up truck at a construction site. The device was sent to the manufacturer for repairs. Leak tests were conducted. No leaks were detected. |
| WNS3 | Aug. 14 | 0 | Packaging and transport | Waste nuclear substance | A package received was misclassified as “Excepted” and should have been classified as “Type A” with respect to IAEA regulations. There was no impact on the health and safety of workers, the public or the environment as a result of this event. |
| 3126 | Aug. 14 | 0 | Malfunctioning device | Industrial | The source rod and shutter on a portable gauge were malfunctioning. The gauge was repaired. |
| 3128 | Aug. 17 | 0 | Unplanned exposure | Industrial | A non-NEW received a dose below regulatory limits upon entering an area wherE industrial radiography was being performed. |
| 3129 | Aug. 16 | 0 | Unplanned exposure | Industrial | A portable gauge was transported with the shutter stuck in the open position. The device was repaired. Doses received as a result of the incident were below regulatory limits. |
| 3130 | Aug. 21 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. The portable gauge was not damaged. Leak tests were performed. No leaks were detected. |
| 3131 | Aug. 21 | 0 | Breach of security | Industrial | A break-in occurred at a licensee’s facility. Neither the locks protecting the radioactive sources nor the building housing them was damaged. No radioactive sources were taken. |
| 3133 | Aug. 21 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by a bulldozer at a construction site. The source remained in the shielded position. The gauge was properly packaged and sent to the manufacturer for disposal. |
| 3136 | Aug. 24 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. Neither the Type A package nor the portable gauge was damaged as a result of the incident. Leak tests were conducted. No eaks were detected. |
| 3134 | Aug. 25 | 0 | Breach of security | Academic and research | A maintenance contractor accessed a storage area for radioactive materials without the knowledge or consent of the RSO. The door to the area was left unlocked by the contractor. A full inventory verification was conducted following the event; all radioactive material was accounted for. |
| 3138 | Aug. 30 | 0 | Breach of security | Industrial | A break-in occurred at a licensee’s location. The building where the radioactive sources are stored was not touched and none of the alarm systems on that building were activated. No nuclear substances went missing. This was the second break-in in a month at this location. |
| 3150 | Aug. 31 | 0 | Malfunctioning device | Industrial | The screw cap covering the americium-241 source in a portable gauge was unscrewed. The service technician repaired the cap. |
| 3143 | Sep. 3 | 0 | Breach of security | Commercial | There was an attempted break-in at a licensee’s location. No nuclear substances were missing. |
| 3140 | Sep. 5 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. The portable gauge was not damaged. Leak tests were performed. No leaks were detected. |
| 3144 | Sep. 6 | 0 | Malfunctioning device | Industrial | The shutter of a fixed gauge was stuck in the open position. The device was repaired. |
| 3148 | Sep. 8 | 0 | Contamination | Academic and research | A package containing sulfur-35 was damaged during transport and resulted in most of the contents spilling on the vial. A non-NEW received doses that were below the regulatory limits as a result of this event. |
| 3152 | Sep. 11 | 0 | Spill | Commercial | A spill of iodine-131 occurred in an isotope processing facility when a bottle tipped of a cart. The affected area was decontaminated. There were no releases to the environment as a result of the incident. The licensee did not provide any information regarding corrective actions to prevent recurrence. |
| 3221 | Sep. 11 | 0 | Packaging and transport | Industrial | A Type A package containing a portable gauge was punctured during transport. There was no damage to the portable gauge itself. |
| 3149 | Sep. 12 | 0 | Damaged device | Industrial | A portable gauge was damaged at a construction site when it was hit by a backhoe. The source remained in the shielded position. The gauge was transported to a service provider for repair. Leak tests were conducted. No leaks were detected. |
| 3151 | Sep. 16 | 0 | Damaged device | Industrial | The shutter handle of a fixed gauge mounted on a pipe broke off as a result of vibrations. Radiation surveys indicated dose rates were in the normal range. The gauge was removed from the work location and transported to safe storage until it could be repaired or disposed of by a service provider. |
| 3153 | Sep. 19 | 0 | Spill | Commercial | A spill of iodine-131 occurred in a laboratory as a result of a technician using the wrong tool to handle the vial. The affected area was decontaminated. |
| 3156 | Sep. 25 | 0 | Spill | Commercial | A vial containing iodine‑131 broke, resulting in a spill contained in a shielded manufacturing box. The affected box was left to decay. There were no releases to the environment or overexposures as a result of the incident. |
| 3157 | Sep. 27 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was dropped approximately 1.2 m. The source remained in the shielded position. The device was sent to a service provider for repair. Leak tests were performed. No leaks were detected. |
| 3158 | Sep. 28 | 0 | Damaged device | Industrial | The source rod handle of a portable gauge broke off the source rod due to metal fatigue. The source was in the shielded position at the time of the incident. The device was transferred to a service provider for disposal. |
| 3159 | Oct. 1 | 1 | Missing or found | Industrial | A portable gauge was stolen from the back of a vehicle overnight. The device has not been recovered. |
| 3220 | Oct. 1 | 0 | Missing or found | Academic and research | A Pb-210 sealed source (Category 5) used for teaching was lost. There is no reason to suspect theft. The source was not found. |
| 3160 | Oct. 2 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was hit by a trailer at a construction site. Radiation surveys indicated that the dose rate was a normal level. The gauge was transferred to a service provider for disposal. |
| 3164 | Oct. 5 | 0 | Missing or found | Academic and research | A sealed source used for calibration of a portal monitor was left unattended within the licensee’s protected area. There were no overexposures as a result of the incident. |
| 3165 | Oct. 12 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. There was no damage to the portable gauge or its Type A package. Leak tests were conducted. No leaks were detected. |
| 3167 | Oct. 13 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. There was no damage to the portable gauge or its Type A package. Leak tests were conducted. No leaks were detected. |
| 3168 | Oct. 16 | 0 | Unplanned exposure | Industrial | A portable gauge was transported with an open shutter. |
| 3245 | Oct. 18 | 0 | Damaged device | Industrial | A portable gauge was damaged by heavy equipment at a construction site. The source rod was damaged and could not be retracted into its shielded position. The device was packaged in a drum and transported back to the licensee’s facility until arrangements could be made for its disposal. |
| 3172 | Oct. 23 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by a construction vehicle. The operator was able to retract the source rod into the shielded position. The device was sent to a service provider for repair. Leak tests were conducted. No leaks were detected. |
| 3175 | Oct. 27 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by heavy equipment at a construction site. The source remained in the shielded position. The gauge was transferred to a third party for disposal. |
| 3176 | Oct. 27 | 0 | Unplanned exposure | Academic and research | A NEW working with fluorine‑18 in a hot cell without all of the necessary personal protective equipment received skin contamination to the wrist. There were no overexposures as a result of this event. |
| 3181 | Oct. 30 | 0 | Packaging and transport | Industrial | A vehicle transporting a portable gauge was involved in a collision. Neither the portable gauge nor its Type A package were damaged. Leak tests were performed. No leaks were detected. |
| 3180 | Nov. 1 | 0 | Missing or found | Medical | A Category 5 sealed source was lost at a hospital. It has not been recovered. |
| 3187 | Nov. 9 | 0 | Damaged device | Industrial | A guide rod of a portable gauge broke due to age. The source remained in the shielded position. Leak tests were conducted. No leaks were detected. The gauge was taken out of service and was ultimately transferred to a service provider for disposal. |
| 3193 | Nov. 9 | 0 | Damaged device | Industrial | The source could not be retracted into the shielded position on a fixed gauge. The licensee stopped the process line where the gauge was located. The source could then be put into the shielded position. The device was removed and placed in storage. |
| 3194 | Nov. 10 | 0 | Damaged device | Industrial | Stress cracks were found in the source holder housing for a fixed gauge. Arrangements have been made to replace the gauge. |
| 3196 | Nov. 16 | 0 | Missing or found | Medical | A Category 5 sealed source (iodine‑125) used in a medical procedure was lost following its removal from a patient. It has not been recovered. |
| 3214 | Nov. 19 | 0 | Malfunctioning device | Industrial | The remote control crank handle of an industrial radiography exposure device was separated from the remote control when the source was exposed. The handle was repaired and the source was retracted to the shielded position. |
| 3198 | Nov. 20 | 0 | Packaging and transport | Commercial | A package containing medical isotopes was delivered to the wrong address. The package was recovered and returned to the consignor. |
| WNS5 | Nov. 20 | 0 | Breach of security | Waste nuclear substance | An attempted break-in occurred, during which an attempt was made to cut through the perimeter fence. Repairs were made to the fence, and no personnel or other equipment was affected. The nuclear substances were not affected. |
| 3199 | Nov. 23 | 0 | Breach of security | Industrial | An individual in an unmarked vehicle entered a licensee’s rail yard without obtaining proper authorization. The individual was escorted off the property. There was no danger to the fixed gauges onsite. |
| 3200 | Nov. 23 | 0 | Packaging and transport | Commercial | A vehicle transporting packages containing nuclear medicine isotopes was involved in a collision. The packages were not damaged. |
| 3204 | Nov. 23 | 0 | Unplanned exposure | Industrial | Two NEWs conducting industrial radiography work ignored their personal dosimetry alarms and approached the exposure device while the source was in the exposed position. The workers were suspended pending investigation. The doses received as a result of the event were below regulatory limits. |
| 3207 | Nov. 23 | 0 | Spill | Medical | A spill of fluorine-18 occurred in a hot room. No overexposures occurred as a result of this event. |
| 3210 | Dec. 4 | 0 | Unplanned exposure | Industrial | A portable gauge was transported with an open shutter. Dose limits received as a result of this occurrence were below regulatory limits. |
| 3212 | Dec. 5 | 0 | Packaging and transport | Commercial | A vehicle transporting packages containing nuclear medicine isotopes was involved in a collision. The packages were not damaged. |
| 3218 | Dec. 11 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was hit by heavy equipment at a construction site. The source remained in the shielded position. The device was transferred to a service provider for disposal. |
| 3219 | Dec. 13 | 0 | Damaged device | Industrial | A portable gauge was damaged when it was run over by heavy equipment at a construction site. The source remained in the shielded position. The device was transferred to a service provider for disposal. |
| 3224 | Dec. 13 | 0 | Packaging and transport | Commercial | Three packages containing isotopes for nuclear medicine were delivered to the wrong address. The shipper corrected the error. |
| 3223 | Dec. 17 | 0 | Missing or found | Industrial | A worker for a construction site found a fixed gauge on the side of a highway. The licensee was contacted and retrieved the gauge. |
| 3231 | Dec. 18 | 0 | Malfunctioning device | Industrial | The crank of an exposure device was not functioning properly and resulted in a source disconnect. |
| 3226 | Dec. 19 | 0 | Missing or found | Historical | A load of scrap metal originating from a landfill triggered the portal alarm at a scrap metal facility. Further investigation determined that radium aircraft dials were in the load of metal. These were removed and stored in a secure location until arrangements can be made for their disposal through the Historic Waste Program Management Office, formerly the Low-Level Radioactive Waste Management Office. |
| 3227 | Dec. 20 | 0 | Missing or found | Medical | An iodine-125 sealed source (brachyseed) used in cancer treatment was lost. This is a Category 5 source. The source was not recovered. |
Appendix D: Inspections conducted in 2017
| Inspection Date | Licensee Name | City | Province | Inspection Type | Sector |
|---|---|---|---|---|---|
| 4-Jan | Golder Associates Ltd. | Mississauga | ON | Type II | Industrial |
| 5-Jan | Toronto Research Chemicals Inc. | North York | ON | Type II | Commercial |
| 5-Jan | Irving Consumer Products Limited | Toronto | ON | Type II | Industrial |
| 5-Jan | Honeywell Ltd | Toronto | ON | Type II | Commercial |
| 6-Jan | Pro-Lab Diagnostics Inc. | Richmond Hill | ON | Type II | Academic and research |
| 8-Jan | K.V. Inspection Services Ltd. | Oakville | ON | Type II | Industrial |
| 9-Jan | Nasiruddin Engineering Limited | Mississauga | ON | Type II | Industrial |
| 9-Jan | Canadian Dewatering (2006) Ltd. | Edmonton | AB | Type II | Industrial |
| 9-Jan | Tuboscope Vetco Canada ULC | Nisku | AB | Type II | Industrial |
| 9-Jan | Tier 1 Energy Solutions, Inc. | Leduc | AB | Type II | Industrial |
| 10-Jan | University of Alberta | Edmonton | AB | Type II | Academic and research |
| 10-Jan | EFW Radiology | Calgary | AB | Type II | Medical |
| 10-Jan | A & A Concrete X-Ray and Coring Ltd. | Surrey | BC | Type II | Industrial |
| 10-Jan | Di-Tech Inc | Montréal | QC | Type II | Industrial |
| 10-Jan | St. Joseph’s Health Care, London | London | ON | Type II | Medical |
| 10-Jan | St. Joseph’s Health Care, London | London | ON | Type II | Medical |
| 10-Jan | St. Joseph’s Health Care, London | London | ON | Type II | Medical |
| 10-Jan | St. Joseph’s Health Care, London | London | ON | Type II | Medical |
| 10-Jan | 860851 Alberta Ltd. | Edmonton | AB | Type II | Industrial |
| 10-Jan | Candu Inspection Inc. | New Norway | AB | Type II | Industrial |
| 10-Jan | Entreprise Gestion Indorama Inc. | Montréal | QC | Type II | Industrial |
| 10-Jan | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Edmonton | AB | Type II | Industrial |
| 10-Jan | Halliburton Canada | Nisku | AB | Type II | Industrial |
| 10-Jan | Halliburton Canada | Nisku | AB | Type II | Industrial |
| 10-Jan | React Radiography Ltd. | Edmonton | AB | Type II | Industrial |
| 10-Jan | 1068648 B.C. Ltd. | Surrey | BC | Type II | Industrial |
| 11-Jan | Steel Inspection & Testing Ltd. | St Catharines | ON | Type II | Industrial |
| 11-Jan | Insight Medical Holdings Ltd. | Edmonton | AB | Type II | Medical |
| 11-Jan | BAKOSNDT Ltd. | Drayton Valley | AB | Type II | Industrial |
| 11-Jan | ITL Testing Laboratories Ltd. | Maple Ridge | BC | Type II | Industrial |
| 11-Jan | 860851 Alberta Ltd. | Drayton Valley | AB | Type II | Industrial |
| 11-Jan | Philips Electronics Ltd./Philips Electronique Ltée | Markham | ON | Type II | Commercial |
| 11-Jan | Philips Electronics Ltd./Philips Electronique Ltée | Markham | ON | Type II | Commercial |
| 11-Jan | TISI Canada Inc. | Edmonton | AB | Type II | Industrial |
| 11-Jan | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Surrey | BC | Type II | Industrial |
| 11-Jan | Guelph General Hospital | Guelph | ON | Type II | Medical |
| 11-Jan | Trenergy Inc. | St Catharines | ON | Type II | Industrial |
| 11-Jan | Valley Geotechnical Engineering Services Ltd. | Langley | BC | Type II | Industrial |
| 12-Jan | Northern Alberta Institute of Technology | Edmonton | AB | Type II | Industrial |
| 12-Jan | Shaw Pipeline Services Ltd. | Sherwood Park | AB | Type II | Industrial |
| 12-Jan | Acuren Inc. | Edmonton | AB | Type II | Industrial |
| 13-Jan | Allnorth Consultants Limited | Sylvan Lake | AB | Type II | Industrial |
| 13-Jan | Superior General Partner Inc. | North Vancouver | BC | Type II | Industrial |
| 13-Jan | UTC Fire & Security Canada Inc. operating as Chubb Edwards | Edmonton | AB | Type II | Commercial |
| 13-Jan | Sunnybrook Health Sciences Centre | Toronto | ON | Type II | Commercial |
| 16-Jan | Echo NDE Inc. | Red Deer | AB | Type II | Industrial |
| 16-Jan | INEOS Canada Company | Joffre | AB | Type II | Industrial |
| 16-Jan | HSPP General Partner Ltd. | Port Mellon | BC | Type II | Industrial |
| 16-Jan | Coca-Cola Refreshments Canada Company/ | Brampton | ON | Type II | Industrial |
| 16-Jan | Coca-Cola Refreshments Canada Company/ | Weston | ON | Type II | Industrial |
| 17-Jan | Kubota Materials Canada Corporation | Orillia | ON | Type II | Industrial |
| 17-Jan | DGI Geoscience Inc. | Barrie | ON | Type II | Industrial |
| 17-Jan | Scanning Technologies Inc. | Edmonton | AB | Type II | Industrial |
| 17-Jan | Metalogic Inspection Services Inc. | Edmonton | AB | Type II | Industrial |
| 17-Jan | JML Biopharm Inc. | North Vancouver | BC | Type II | Academic and research |
| 17-Jan | Wakefield Canada Inc. | Toronto | ON | Type II | Industrial |
| 17-Jan | Revolution Acquisition GP Inc. | North Vancouver | BC | Type II | Industrial |
| 17-Jan | Alberta Health Services | Edmonton | AB | Type I | Medical |
| 18-Jan | Vancouver Coastal Health Authority | Surrey | BC | Type II | Medical |
| 18-Jan | Vancouver Coastal Health Authority | Surrey | BC | Type II | Medical |
| 18-Jan | EFW Radiology | Calgary | AB | Type II | Medical |
| 18-Jan | Selenis Canada Inc. | Montréal | QC | Type II | Industrial |
| 18-Jan | Provincial Health Services Authority (British Columbia) | Surrey | BC | Type II | Medical |
| 18-Jan | Titanium Tubing Technology Ltd. | Lloydminster | AB | Type II | Industrial |
| 18-Jan | Construction DJL Inc./ | Montréal | QC | Type II | Industrial |
| 19-Jan | BWXT Canada LTD. | Cambridge | ON | Type II | Industrial |
| 19-Jan | Perfection Inspection Limited | Cambridge | ON | Type II | Industrial |
| 19-Jan | Perfection Inspection Limited | Cambridge | ON | Type II | Industrial |
| 19-Jan | Unique Detection Services Limited | Cambridge | ON | Type II | Industrial |
| 19-Jan | Vibac Canada Inc. | Montréal | QC | Type II | Industrial |
| 19-Jan | Polar Plastique Ltée | Saint-Laurent | QC | Type II | Industrial |
| 19-Jan | MPE Engineering Ltd. | Medicine Hat | AB | Type II | Industrial |
| 19-Jan | Shell Global Solutions Canada Inc. | Calgary | AB | Type II | Industrial |
| 19-Jan | GHD Consultants Ltd. | Waterloo | ON | Type II | Industrial |
| 19-Jan | Schlumberger Canada Limited | Medicine Hat | AB | Type II | Industrial |
| 19-Jan | Shell Canada Limited | Calgary | AB | Type II | Industrial |
| 20-Jan | Alberta Health Services | Edmonton | AB | Type II | Medical |
| 20-Jan | Medicine Hat Regional Hospital | Medicine Hat | AB | Type II | Medical |
| 20-Jan | Medicine Hat Regional Hospital | Medicine Hat | AB | Type II | Medical |
| 23-Jan | Dart Canada Inc. | Scarborough | ON | Type II | Industrial |
| 23-Jan | Englobe Corp. | Calgary | AB | Type II | Industrial |
| 23-Jan | EXP Services Inc. | Oromocto | NB | Type II | Industrial |
| 24-Jan | Regional Health Authority B | Fredericton | NB | Type II | Medical |
| 24-Jan | Regional Health Authority B | Fredericton | NB | Type II | Medical |
| 24-Jan | Conquest Engineering Ltd. | Fredericton | NB | Type II | Industrial |
| 24-Jan | BCG Engineering Inc. | Fredericton | NB | Type II | Industrial |
| 25-Jan | NB Department of Transportation and Infrastructure | Andover | NB | Type II | Industrial |
| 25-Jan | Wilfrid Laurier University | Waterloo | ON | Type II | Academic and research |
| 25-Jan | Lascelles Engineering and Associates Ltd. | Hawkesbury | ON | Type II | Industrial |
| 25-Jan | AV Group NB Inc. | Nackawic | NB | Type II | Industrial |
| 26-Jan | ABB Inc. | Saint-Laurent | QC | Type II | Commercial |
| 26-Jan | Englobe Corp. | Anjou | QC | Type II | Industrial |
| 26-Jan | Englobe Corp. | Stratford | ON | Type II | Industrial |
| 26-Jan | Englobe Corp. | Toronto | ON | Type II | Industrial |
| 26-Jan | Polyfilm Extrusions Ltd. | Montréal | QC | Type II | Industrial |
| 26-Jan | Geolog Solutions Inc. | Red Deer County | AB | Type II | Industrial |
| 26-Jan | Halliburton Canada | Red Deer | AB | Type II | Industrial |
| 28-Jan | Schlumberger Canada Limited | Nisku | AB | Type II | Industrial |
| 30-Jan | Uni-Tech Inspection Services Ltd. | South Glengarry | ON | Type II | Industrial |
| 30-Jan | Uni-Tech Inspection Services Ltd. | South Glengarry | ON | Type II | Industrial |
| 30-Jan | Englobe Corp. | Edmonton | AB | Type II | Industrial |
| 30-Jan | Canadian Tower Scanning Inc. | Sarnia | ON | Type II | Industrial |
| 30-Jan | Isologic Innovative Radiopharmaceuticals Ltd. | Dorval | QC | Type I | Commercial |
| 31-Jan | Children’s Hospital of Eastern Ontario | Ottawa | ON | Type II | Medical |
| 31-Jan | Children’s Hospital of Eastern Ontario | Ottawa | ON | Type II | Academic and research |
| 31-Jan | Children’s Hospital of Eastern Ontario | Ottawa | ON | Type II | Medical |
| 31-Jan | Clear Image Inspection Ltd. | Bentley | AB | Type II | Industrial |
| 31-Jan | Welltec Canada Inc. | Stettler | AB | Type II | Industrial |
| 1-Feb | Core Laboratories Canada Ltd. | Red Deer | AB | Type II | Industrial |
| 1-Feb | Galey Inspection Services Ltd. | Carvel | AB | Type II | Industrial |
| 1-Feb | NOVA Chemicals Corporation | Joffre | AB | Type II | Industrial |
| 2-Feb | Cascades Canada ULC | Scarborough | ON | Type II | Industrial |
| 2-Feb | University of Guelph | Guelph | ON | Type II | Medical |
| 2-Feb | University of Guelph | Guelph | ON | Type II | Academic and research |
| 2-Feb | University of Guelph | Guelph | ON | Type II | Commercial |
| 2-Feb | Ontario Power Generation Inc. | Calabogie | ON | Type II | Academic and research |
| 2-Feb | Quadrant Plastic Composites Canada Inc. | Guelph | ON | Type II | Industrial |
| 2-Feb | Philips Lighting Canada Ltd. | Markham | ON | Type II | Commercial |
| 2-Feb | UTQUALITY INC. | Edmonton | AB | Type II | Industrial |
| 2-Feb | Pylon Electronics Inc. | Ottawa | ON | Type II | Commercial |
| 2-Feb | 5N Plus Inc. | Saint-Laurent | QC | Type II | Industrial |
| 3-Feb | Weatherford Canada Ltd. | Nisku | AB | Type II | Industrial |
| 4-Feb | Accuray Inc. | Ottawa | ON | Type II | Commercial |
| 6-Feb | All Test International Inc. | Brooks | AB | Type II | Industrial |
| 6-Feb | Boss Wireline Services Ltd. | Brooks | AB | Type II | Industrial |
| 7-Feb | All Test International Inc. | Medicine Hat | AB | Type II | Industrial |
| 7-Feb | Express Pipeline Ltd. | Medicine Hat | AB | Type II | Industrial |
| 7-Feb | Southlake Regional Health Centre | Newmarket | ON | Type II | Medical |
| 8-Feb | C.B. Non-Destructive Testing Ltd | Oakville | ON | Type II | Industrial |
| 8-Feb | AR Geotechnical Engineering Ltd. | Medicine Hat | AB | Type II | Industrial |
| 8-Feb | National Research Council of Canada | Ottawa | ON | Type II | Academic and research |
| 8-Feb | National Research Council of Canada | Ottawa | ON | Type II | Academic and research |
| 8-Feb | National Research Council of Canada | Ottawa | ON | Type II | Academic and research |
| 8-Feb | National Research Council of Canada | Ottawa | ON | Type II | Academic and research |
| 8-Feb | National Research Council of Canada | Ottawa | ON | Type II | Academic and research |
| 9-Feb | Slick Inspection Limited | Medicine Hat | AB | Type II | Industrial |
| 9-Feb | 1788966 Alberta Ltd. | Redcliff | AB | Type II | Industrial |
| 9-Feb | Honeywell Ltd | Lachine | QC | Type II | Commercial |
| 9-Feb | Isologic Innovative Radiopharmaceuticals Ltd. | Toronto | ON | Type II | Commercial |
| 10-Feb | St Lawrence Testing & Inspection Co. Ltd. | Cornwall | ON | Type II | Industrial |
| 10-Feb | Voltage Wireline Inc. | Brooks | AB | Type II | Industrial |
| 13-Feb | Englobe Corp. | Joliette | QC | Type II | Industrial |
| 13-Feb | Englobe Corp. | Calgary | AB | Type II | Industrial |
| 14-Feb | Vancouver Coastal Health Authority | New Westminster | BC | Type II | Medical |
| 14-Feb | Vancouver Coastal Health Authority | New Westminster | BC | Type II | Medical |
| 14-Feb | Englobe Corp. | Drummondville | QC | Type II | Industrial |
| 14-Feb | Provincial Health Services Authority (British Columbia) | New Westminster | BC | Type II | Medical |
| 14-Feb | Lehigh Northwest Cement Limited | Delta | BC | Type II | Industrial |
| 14-Feb | Lakeridge Health | Oshawa | ON | Type I | Medical |
| 14-Feb | Lakeridge Health | Oshawa | ON | Type II | Medical |
| 15-Feb | Englobe Inc. | Laval | QC | Type I | Industrial |
| 15-Feb | Rainbow Engineering Inc. | CAlgary | AB | Type II | Industrial |
| 15-Feb | GeoPacific Consultants Ltd. | Vancouver | BC | Type II | Industrial |
| 15-Feb | Trans Mountain Pipeline ULC | Burnaby | BC | Type II | Industrial |
| 15-Feb | Inception Sciences Canada, inc. | Vancouver | BC | Type II | Academic and research |
| 16-Feb | University of British Columbia | Vancouver | BC | Type II | Academic and research |
| 16-Feb | University of British Columbia | Vancouver | BC | Type II | Academic and research |
| 16-Feb | EFW Radiology | Calgary | AB | Type II | Medical |
| 16-Feb | EFW Radiology | Calgary | AB | Type II | Medical |
| 16-Feb | Signalchem Pharmaceuticals Inc. | Richmond | BC | Type II | Academic and research |
| 17-Feb | Vancouver General Hospital | Vancouver | BC | Type II | Commercial |
| 20-Feb | Sintra Inc. | Bécancour | QC | Type II | Industrial |
| 20-Feb | Canadoil Forge Ltée/Canadoil Forge Ltd. | Bécancour | QC | Type II | Industrial |
| 21-Feb | Nova Scotia Health Authority | Halifax | NS | Type II | Medical |
| 21-Feb | Cascades Canada ULC | Cap-de-la-Madeleine | QC | Type II | Industrial |
| 21-Feb | Ontario Power Generation Inc. | Bowmanville | ON | Type II | Industrial |
| 21-Feb | Triquest Nondestructive Testing Corp. | Calgary | AB | Type II | Industrial |
| 21-Feb | Kinectrics Inc. | Toronto | ON | Type II | Academic and research |
| 21-Feb | Kinectrics Inc. | Toronto | ON | Type II | Academic and research |
| 21-Feb | Excavation Daniel Latour Inc. | Lavaltrie | QC | Type II | Industrial |
| 21-Feb | Sirati & Partners Consultants Ltd. | Vaughan | ON | Type II | Industrial |
| 21-Feb | EXP Services Inc. / Les Services EXP Inc. | Trois-Rivières | QC | Type II | Industrial |
| 21-Feb | Nova Scotia Health Authority | Halifax | NS | Type II | Medical |
| 21-Feb | Nova Scotia Health Authority | Halifax | NS | Type I | Commercial |
| 23-Feb | Englobe Corp. | Shawinigan | QC | Type II | Industrial |
| 23-Feb | Englobe Corp. | Shawinigan | QC | Type II | Industrial |
| 23-Feb | Troxler Canada Inc. | Mississauga | ON | Type II | Commercial |
| 23-Feb | Certified Testing Systems (2009) Inc. | Kitchener | ON | Type II | Industrial |
| 23-Feb | The Graff Company Ltd. | Mississauga | ON | Type II | Industrial |
| 23-Feb | Centre Intégré Universitaire de santé et de services sociaux | Shawinigan-Sud | QC | Type II | Medical |
| 23-Feb | Centre Intégré Universitaire de santé et de services sociaux | Shawinigan-Sud | QC | Type II | Medical |
| 24-Feb | Canadian Institute for NDE | Hamilton | ON | Type II | Industrial |
| 24-Feb | LifeLabs Inc. | Toronto | ON | Type II | Academic and research |
| 24-Feb | Compagnie Westrock Du Canada Inc. | La Tuque | QC | Type II | Industrial |
| 24-Feb | Brampton Engineering Inc. | Brampton | ON | Type II | Industrial |
| 27-Feb | Layfield Canada Ltd. | Richmond | BC | Type II | Industrial |
| 27-Feb | Inteplast Bags and Films Corporation | Lanoraie d’Autray | QC | Type II | Industrial |
| 27-Feb | Canadian Cutting & Coring (Toronto) Ltd | Mississauga | ON | Type II | Industrial |
| 28-Feb | Université Concordia/ Concordia University | Montréal | QC | Type II | Medical |
| 28-Feb | E.F. Monk Holdings Limited | Dartmouth | NS | Type II | Industrial |
| 28-Feb | Big Guns Energy Services Inc. | Red Deer | AB | Type II | Industrial |
| 28-Feb | TISI Canada Inc. | Dartmouth | NS | Type II | Industrial |
| 28-Feb | Custom Fabricators & Machinists Limited / Fabricants et Mach | Dartmouth | NS | Type II | Industrial |
| 28-Feb | Omnifission Inc. | Brampton | ON | Type II | Commercial |
| 28-Feb | Whistler Water Inc. | Burnaby | BC | Type II | Industrial |
| 1-Mar | Simon Fraser University | Burnaby | BC | Type II | Academic and research |
| 1-Mar | Nova Scotia Health Authority | Bridgewater | NS | Type II | Medical |
| 1-Mar | Ezeflow Inc. | Granby | QC | Type II | Industrial |
| 1-Mar | WSP Canada Inc. | Langley | BC | Type II | Industrial |
| 1-Mar | Harbourside Geotechnical Consultants Limited | Dartmouth | NS | Type II | Industrial |
| 1-Mar | EXP Services Inc. | Calgary | AB | Type II | Industrial |
| 2-Mar | KMH Cardiology Centres Incorporated | Mississauga | ON | Type II | Medical |
| 2-Mar | KMH Cardiology Centres Incorporated | Mississauga | ON | Type II | Medical |
| 2-Mar | Nova Scotia Health Authority | Kentville | NS | Type II | Medical |
| 2-Mar | Resolute FP Canada Inc. / PF Résolu Canada Inc. | Jonquière | QC | Type II | Industrial |
| 2-Mar | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Dartmouth | NS | Type II | Industrial |
| 2-Mar | Mistras Services Inc. | Sacré-Coeur-Saguenay | QC | Type II | Industrial |
| 2-Mar | Vertex Pharmaceuticals (Canada) Incorporated | Laval | QC | Type II | Academic and research |
| 3-Mar | KMH Cardiology Centres Incorporated | Hamilton | ON | Type II | Medical |
| 3-Mar | KMH Cardiology Centres Incorporated | Woodstock | ON | Type II | Medical |
| 6-Mar | Molson Canada 2005 | Moncton | NB | Type II | Industrial |
| 6-Mar | PML Inspection Services Ltd. | Fort Saskatchewan | AB | Type II | Industrial |
| 6-Mar | EXP Services Inc. | Moncton | NB | Type II | Industrial |
| 6-Mar | Regional Health Authority B | Moncton | NB | Type II | Medical |
| 6-Mar | Regional Health Authority B | Moncton | NB | Type II | Medical |
| 6-Mar | Nyrstar Myra Falls Inc. | Campbell River | BC | Type II | Industrial |
| 6-Mar | Dr. William Bruce Taylor | Edmonton | AB | Type I | Medical |
| 7-Mar | RTD Quality Services Inc. | Bathurst | NB | Type II | Industrial |
| 7-Mar | Conquest Engineering Ltd. | Moncton | NB | Type II | Industrial |
| 7-Mar | Neucel Specialty Cellulose Ltd. | Port Alice | BC | Type II | Industrial |
| 7-Mar | TransAlta Utilities Corporation | Duffield | AB | Type II | Industrial |
| 7-Mar | TransAlta Utilities Corporation | Duffield | AB | Type II | Industrial |
| 7-Mar | TransAlta Utilities Corporation | Duffield | AB | Type II | Industrial |
| 7-Mar | 1583023 Alberta Ltd. | Whitecourt | AB | Type II | Industrial |
| 7-Mar | Mistras Services Inc. | Terrebonne | QC | Type II | Industrial |
| 7-Mar | Mistras Services Inc. | Saint-Lambert | QC | Type II | Industrial |
| 7-Mar | Focus NDTIS Inc. | Edmonton | AB | Type II | Industrial |
| 7-Mar | TISI Canada Inc. | Dartmouth | NS | Type II | Industrial |
| 7-Mar | Dalhousie University | Halifax | NS | Type II | Industrial |
| 8-Mar | TJ Inspection Services | Dartmouth | NS | Type II | Industrial |
| 8-Mar | Ground Engineering & Materials Consultants Ltd. | Saint John | NB | Type II | Industrial |
| 8-Mar | Potash Corporation of Saskatchewan Inc. | Penobsquis | NB | Type II | Industrial |
| 8-Mar | Nanaimo Forest Products Ltd. | Nanaimo | BC | Type II | Industrial |
| 8-Mar | Acuren Inc. | Nanaimo | BC | Type II | Industrial |
| 8-Mar | Hoskin Scientific Limited | St-Laurent | QC | Type II | Commercial |
| 8-Mar | Hoskin Scientific Limited | St-Laurent | QC | Type II | Commercial |
| 9-Mar | Regional Health Authority B | Saint John | NB | Type II | Commercial |
| 9-Mar | Regional Health Authority B | Saint John | NB | Type II | Medical |
| 9-Mar | Regional Health Authority B | Saint John | NB | Type II | Medical |
| 9-Mar | Lafarge Canada Inc. | St-Constant | QC | Type II | Industrial |
| 9-Mar | University of British Columbia | Bamfield | BC | Type II | Academic and research |
| 9-Mar | Atlantic Packaging Products Ltd. | Whitby | ON | Type II | Industrial |
| 9-Mar | University of Alberta | Bamfield | BC | Type II | Academic and research |
| 9-Mar | Western Canadian Universities Marine Sciences Society | Bamfield | BC | Type II | Academic and research |
| 9-Mar | Western Canadian Universities Marine Sciences Society | Bamfield | BC | Type II | Academic and research |
| 9-Mar | Irving Paper | Saint John | NB | Type II | Industrial |
| 9-Mar | Ajax Textile Corporation | Ajax | ON | Type II | Industrial |
| 9-Mar | Custom Fabricators & Machinists Limited | Saint John | NB | Type II | Industrial |
| 9-Mar | Canadian Blood Services/ Société canadienne du sang | Saint John | NB | Type II | Medical |
| 10-Mar | Alberta Health Services | Calgary | AB | Type II | Commercial |
| 10-Mar | EXP Services Inc. | Saint John | NB | Type II | Industrial |
| 10-Mar | Tetra Tech EBA Inc. | Nanaimo | BC | Type II | Industrial |
| 10-Mar | Gerdau Ameristeel Cambridge Inc. | Cambridge | ON | Type II | Industrial |
| 10-Mar | Barrday, Inc. | Cambridge | ON | Type II | Industrial |
| 10-Mar | Streamline Inspection Limited | Red Deer | AB | Type II | Industrial |
| 10-Mar | Kodiak Nondestructive Testing Services Ltd. | Nanaimo | BC | Type II | Industrial |
| 13-Mar | Institut national de la recherche scientifique | Québec | QC | Type II | Academic and research |
| 13-Mar | Construction & Pavage Portneuf Inc. | St-Marc-Carrières | QC | Type II | Industrial |
| 13-Mar | Alberta Health Services | Calgary | AB | Type II | Medical |
| 13-Mar | Alberta Health Services | Calgary | AB | Type II | Medical |
| 14-Mar | Eagle Engineering Corp. | Bragg Creek | AB | Type II | Industrial |
| 14-Mar | Alberta Health Services | Calgary | AB | Type II | Medical |
| 14-Mar | Alberta Health Services | Calgary | AB | Type II | Medical |
| 14-Mar | SNC-Lavalin GEM Québec Inc. | Québec | QC | Type II | Industrial |
| 14-Mar | Mistras Services Inc. | Lévis | QC | Type II | Industrial |
| 14-Mar | Mistras Services Inc. | Lévis | QC | Type II | Industrial |
| 14-Mar | Mistras Services Inc. | Lévis | QC | Type II | Industrial |
| 14-Mar | Mistras Services Inc. | Lévis | QC | Type II | Industrial |
| 15-Mar | Kronos Canada, Inc. | Varennes | QC | Type II | Industrial |
| 15-Mar | Solmax International Inc. | Varennes | QC | Type II | Industrial |
| 15-Mar | Spectrum Wireline Services Ltd. | Red Deer County | AB | Type II | Industrial |
| 15-Mar | CHU de Québec - Université Laval | Sainte-Foy | QC | Type II | Medical |
| 15-Mar | CHU de Québec - Université Laval | Sainte-Foy | QC | Type II | Medical |
| 16-Mar | Malpack Ltd. | Ajax | ON | Type II | Industrial |
| 16-Mar | The Pepsi Bottling Group (Canada), ULC | Saint-Laurent | QC | Type II | Industrial |
| 16-Mar | Inteplast Bags and Films Corporation | Vaughan | ON | Type II | Industrial |
| 16-Mar | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Saint-Georges | QC | Type II | Medical |
| 16-Mar | Centre intégré de santé et de services sociaux de Chaudière-Appalaches | Saint-Georges | QC | Type II | Medical |
| 17-Mar | Alberta Health Services | Calgary | AB | Type II | Medical |
| 17-Mar | Alberta Health Services | Calgary | AB | Type II | Medical |
| 20-Mar | Southern Alberta Institute of Technology | Calgary | AB | Type II | Academic and research |
| 20-Mar | Southern Alberta Institute of Technology | Calgary | AB | Type II | Academic and research |
| 20-Mar | ARA Engineering Ltd. | Calgary | AB | Type II | Industrial |
| 20-Mar | Mines D’or Wesdome Inc. | Val d’Or | QC | Type II | Industrial |
| 20-Mar | Kruger Publication Papers Inc./ | Sherbrooke | QC | Type II | Industrial |
| 20-Mar | Graphic Packaging International Canada, ULC | East Angus | QC | Type II | Industrial |
| 21-Mar | Uniboard Canada Inc. | Mont-Laurier | QC | Type II | Industrial |
| 21-Mar | Noremtech Inc. | Stittsville | ON | Type II | Commercial |
| 21-Mar | Noremtech Inc. | Stittsville | ON | Type II | Commercial |
| 21-Mar | Technocell Inc. | Drummondville | QC | Type II | Industrial |
| 21-Mar | Aurora Inspection Limited | Olds | AB | Type II | Industrial |
| 21-Mar | Breakwater Resources Ltd. | Lebel-sur-Quévillon | QC | Type II | Industrial |
| 21-Mar | Mistras Services Inc. | Sherbrooke | QC | Type II | Industrial |
| 21-Mar | Mistras Services Inc. | Sherbrooke | QC | Type II | Industrial |
| 22-Mar | WSP Canada Inc. | Langley | BC | Type II | Industrial |
| 22-Mar | Southern Alberta Institute of Technology | Calgary | AB | Type II | Industrial |
| 22-Mar | Southern Alberta Institute of Technology | Calgary | AB | Type II | Industrial |
| 22-Mar | Québec Lithium Inc. | La Corne | QC | Type II | Industrial |
| 22-Mar | Glencore Canada Corporation | Matagami | QC | Type II | Industrial |
| 23-Mar | Canadian Blood Services/ Société canadienne du sang | Vancouver | BC | Type II | Medical |
| 23-Mar | Les Mines Agnico-Eagle Ltée / Agnico-Eagle Mines Ltd. | Rouyn-Noranda | QC | Type II | Industrial |
| 23-Mar | 2021960 Ontario Inc. O/A New Forest Paper Mills LP | Scarborough | ON | Type II | Industrial |
| 23-Mar | Polytarp Products | Toronto | ON | Type II | Industrial |
| 23-Mar | UTC Fire & Security Canada Inc. operating as Chubb Edwards | Calgary | AB | Type II | Commercial |
| 23-Mar | Higher Ground Consulting Inc. | Calgary | AB | Type II | Industrial |
| 23-Mar | Schlumberger Canada Limited | Red Deer | AB | Type II | Industrial |
| 27-Mar | Les Laboratoires d’Essais Mequaltech Inc. | Montréal | QC | Type II | Industrial |
| 27-Mar | Tracerco Radioactive Diagnostic Services Canada, Inc. | Sarnia | ON | Type II | Industrial |
| 27-Mar | Tracerco Radioactive Diagnostic Services Canada, Inc. | Sarnia | ON | Type II | Industrial |
| 27-Mar | Tracerco Radioactive Diagnostic Services Canada, Inc. | Sarnia | ON | Type II | Commercial |
| 27-Mar | Tracerco Radioactive Diagnostic | Sarnia | ON | Type II | Commercial |
| 27-Mar | Pavages Maska Inc. | St-Hubert | QC | Type II | Industrial |
| 27-Mar | Centre intégré universitaire de santé et de services sociaux | Montréal | QC | Type II | Medical |
| 27-Mar | Centre intégré universitaire de santé et de services sociaux | Montréal | QC | Type II | Medical |
| 27-Mar | Cal Frac Well Services Ltd. | Red Deer | AB | Type II | Industrial |
| 28-Mar | Institut de Cardiologie de Montréal | Montréal | QC | Type II | Medical |
| 28-Mar | Université de Montréal | St-Hyacinthe | QC | Type II | Medical |
| 28-Mar | Tomlinson Enterprises Ltd. | Sarnia | ON | Type II | Industrial |
| 28-Mar | NCL Envirotek Inc. | St-Roch-de-l’Achigan | QC | Type II | Industrial |
| 28-Mar | Les Laboratoire de la Montérégie Inc. | St-Hyacinthe | QC | Type II | Industrial |
| 28-Mar | Klohn Crippen Berger Ltd. | Calgary | AB | Type II | Industrial |
| 28-Mar | Elekta Inc. | Altanta | GA | Type II | Commercial |
| 28-Mar | Baker Hughes Canada Company | Sarnia | ON | Type II | Industrial |
| 28-Mar | Baker Hughes Canada Company | Sarnia | ON | Type II | Industrial |
| 29-Mar | IRISNDT Corp. | Calgary | AB | Type II | Industrial |
| 29-Mar | Horton CBI, Limited | Sturgeon County | AB | Type II | Industrial |
| 29-Mar | Institut national de la recherche scientifique | Varennes | QC | Type II | Academic and research |
| 29-Mar | Canada Border Services Agency | Montréal | QC | Type II | Industrial |
| 29-Mar | Les Inspections Thermetco Inc. | Montréal | QC | Type II | Industrial |
| 29-Mar | Matériel de Laboratoire J.G. Inc. | Laval | QC | Type II | Commercial |
| 29-Mar | Matériel de Laboratoire J.G. Inc. | Laval | QC | Type II | Commercial |
| 29-Mar | Troxler Canada Inc. | Laval | QC | Type II | Commercial |
| 29-Mar | Troxler Canada Inc. | Laval | QC | Type II | Commercial |
| 29-Mar | GHD Consultants Ltd. | Saint-Laurent | QC | Type II | Industrial |
| 29-Mar | GHD Consultants Ltd. | Saint-Laurent | QC | Type II | Industrial |
| 29-Mar | GHD Consultants Ltd. | Montréal | QC | Type II | Industrial |
| 29-Mar | GHD Consultants Ltd. | Montréal | QC | Type II | Industrial |
| 30-Mar | PolyExpert Inc. | Laval | QC | Type II | Industrial |
| 30-Mar | Toronto Equine Hospital | Mississauga | ON | Type II | Medical |
| 30-Mar | National Research Council of Canada | Montréal | QC | Type II | Academic and research |
| 31-Mar | Weatherford Canada Ltd. | Red Deer | AB | Type II | Industrial |
| 5-Apr | Canada Border Services Agency | Surrey | BC | Type II | Industrial |
| 5-Apr | Rapiscan Systems Inc. | Surrey | BC | Type II | Commercial |
| 6-Apr | 2956900 Canada Inc. | Chelsea | QC | Type II | Industrial |
| 6-Apr | Associate Veterinary Clinics | Calgary | AB | Type II | Medical |
| 7-Apr | Royal Ottawa Health Care Group 2002 | Ottawa | ON | Type II | Academic and research |
| 7-Apr | The Ottawa Hospital | Ottawa | ON | Type II | Medical |
| 7-Apr | The Ottawa Hospital | Ottawa | ON | Type II | Medical |
| 18-Apr | Capital Power Corporation (Genesee Station) | Warburg | AB | Type II | Industrial |
| 19-Apr | Stuart Hunt & Associates Ltd. | Edmonton | AB | Type II | Commercial |
| 25-Apr | Centre intégré universitaire de santé et de services sociaux du Saguenay - La-Saint-Jean | Chicoutimi | QC | Type II | Medical |
| 25-Apr | Centre intégré universitaire de santé et de services sociaux du Saguenay - La-Saint-Jean | Chicoutimi | QC | Type I | Medical |
| 25-Apr | British Columbia Cancer Agency | Prince George | BC | Type I | Medical |
| 25-Apr | British Columbia Cancer Agency | Prince George | BC | Type II | Medical |
| 27-Apr | Med-Scan X-Ray & Ultrasound Services Ltd. | Maple | ON | Type II | Medical |
| 28-Apr | Concord Steel Centre Ltd. | Woodbridge | ON | Type II | Industrial |
| 28-Apr | Valmet Ltd. | Vaughan | ON | Type II | Commercial |
| 2-May | Forward Engineering & Associates Inc. | Toronto | ON | Type II | Industrial |
| 2-May | Nine Energy Canada Inc. | Red Deer | AB | Type II | Industrial |
| 2-May | Fermar Asphalt Limited | Rexdale | ON | Type II | Industrial |
| 2-May | Sanjel Energy Services Inc. | Red Deer | AB | Type II | Industrial |
| 3-May | McElhanney Consulting Services Ltd. | Edmonton | AB | Type II | Industrial |
| 3-May | North West Redwater Holdings Corp. | Gibbons | AB | Type II | Industrial |
| 4-May | Clifton Associates Ltd. | Calgary | AB | Type II | Industrial |
| 4-May | Trican Well Service Ltd. | Nisku | AB | Type II | Industrial |
| 4-May | Englobe Corp. | Calgary | AB | Type II | Industrial |
| 5-May | Harold Sutherland Construction Ltd. | Kemble | ON | Type II | Industrial |
| 5-May | Q Test Inspection Ltd. | Sylvan Lake | AB | Type II | Industrial |
| 5-May | Lascelles Engineering and Associates Ltd. | Hawkesbury | ON | Type II | Industrial |
| 8-May | H. Manalo Consulting | Winnipeg | MB | Type II | Industrial |
| 9-May | DST Consulting Engineers Inc. | Kenora | ON | Type II | Industrial |
| 9-May | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Saint-Laurent | QC | Type II | Industrial |
| 9-May | Paraza Pharma Inc. | Montréal | QC | Type II | Academic and research |
| 10-May | J.T. Donald Consultants Limited | Markham | ON | Type II | Industrial |
| 10-May | Haddad Geotechnical Inc. | Markham | ON | Type II | Industrial |
| 10-May | DST Consulting Engineers Inc. | Thunder Bay | ON | Type II | Industrial |
| 10-May | 9139-6903 Québec Inc. | Saint-Sauveur | QC | Type II | Industrial |
| 11-May | Confederation College of Applied Arts & Technology | Thunder Bay | ON | Type II | Industrial |
| 11-May | TISI Canada Inc. | Thunder Bay | ON | Type II | Industrial |
| 11-May | Taranis Contracting Group Ltd. | Thunder Bay | ON | Type II | Industrial |
| 11-May | True Grit Engineering Limited | Thunder Bay | ON | Type II | Industrial |
| 11-May | LH North Ltd. | Rosslyn | ON | Type II | Industrial |
| 12-May | Thunder Bay Regional Health Sciences Centre | Thunder Bay | ON | Type II | Academic and research |
| 12-May | Thunder Bay Regional Health Sciences Centre | Thunder Bay | ON | Type II | Academic and research |
| 12-May | Voltage Wireline Inc. | Blackfalds | AB | Type II | Industrial |
| 15-May | Acadia University | Wolfville | NS | Type II | Academic and research |
| 15-May | Nova Scotia Health Authority | Halifax | NS | Type II | Commercial |
| 15-May | Nova Scotia Health Authority | Halifax | NS | Type II | Academic and research |
| 15-May | Labo S.M. Inc. | Longueuil | QC | Type II | Industrial |
| 15-May | Saga Engineering Inc. | Edmonton | AB | Type II | Industrial |
| 15-May | London Health Sciences Centre | London | ON | Type I | Medical |
| 15-May | London Health Sciences Centre | London | ON | Type II | Medical |
| 16-May | Ingétec Inc. | Laval | QC | Type II | Industrial |
| 16-May | Labo S.M. Inc. | Montréal | QC | Type II | Industrial |
| 16-May | Labo S.M. Inc. | Laval | QC | Type II | Industrial |
| 16-May | Almadon Holdings Ltd. | Calgary | AB | Type II | Medical |
| 16-May | Cambium Inc. | Oshawa | ON | Type II | Industrial |
| 16-May | MyHealth Partners Inc. | Whitby | ON | Type II | Medical |
| 16-May | Maxxam Analytics International Corporation | Port Hope | ON | Type II | Academic and research |
| 17-May | Englobe Corp. | Anjou | QC | Type II | Industrial |
| 17-May | Solmatech Inc. | St-Jérôme | QC | Type II | Industrial |
| 17-May | Groupe ABS Inc. | Blainville | QC | Type II | Industrial |
| 17-May | University of Ontario Institute of Technology | Oshawa | ON | Type II | Academic and research |
| 17-May | University of Ontario Institute of Technology | Oshawa | ON | Type II | Academic and research |
| 17-May | SNC-Lavalin GEM Québec Inc. | Longueuil | QC | Type II | Industrial |
| 17-May | GHD Consultants Ltd. | Mississauga | ON | Type II | Industrial |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Kitchener | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | University of Waterloo | Waterloo | ON | Type II | Academic and research |
| 18-May | Groupe ABS Inc. | Montréal | QC | Type II | Industrial |
| 18-May | Groupe ABS Inc. | Montréal | QC | Type II | Industrial |
| 18-May | Groupe ABS Inc. | Montréal | QC | Type II | Industrial |
| 18-May | Groupe ABS Inc. | Blainville | QC | Type II | Industrial |
| 18-May | Groupe ABS Inc. | Blainville | QC | Type II | Industrial |
| 18-May | Groupe Conseil SCT inc. | Montréal | QC | Type II | Industrial |
| 18-May | University of Ontario Institute of Technology | Oshawa | ON | Type II | Academic and research |
| 18-May | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Saint-Laurent | QC | Type II | Industrial |
| 18-May | SNC-Lavalin GEM Québec Inc. | St-Jean-sur-Richelieu | QC | Type II | Industrial |
| 18-May | SNC-Lavalin GEM Québec Inc. | Longueuil | QC | Type II | Industrial |
| 18-May | EXP Services Inc. / Les Services EXP Inc. | Laval | QC | Type II | Industrial |
| 23-May | Alberta Health Services | Edmonton | AB | Type II | Medical |
| 24-May | York X-Ray Management Limited O/A York Radiology Consultants | Willowdale | ON | Type II | Medical |
| 24-May | Intratech Engineering Laboratories Ltd. | Scarborough | ON | Type II | Industrial |
| 24-May | Engtec Consulting Inc. | Vaughan | ON | Type II | Industrial |
| 24-May | Sable Sand Solutions Inc. | Calgary | AB | Type II | Industrial |
| 24-May | Wright Quality Services Inc. | Beaumont | AB | Type II | Industrial |
| 24-May | Canadian Engineering & Inspection Ltd. | Edmonton | AB | Type II | Industrial |
| 25-May | Tier 1 Energy Solutions, Inc. | Leduc | AB | Type II | Industrial |
| 25-May | Baker Hughes Canada Company | Leduc | AB | Type II | Industrial |
| 25-May | Isologic Innovative Radiopharmaceuticals Ltd. | Burlington | ON | Type II | Commercial |
| 25-May | Alberta Health Services | Calgary | AB | Type I | Medical |
| 26-May | Sudbury Neutrino Observatory | Sudbury | ON | Type II | Academic and research |
| 29-May | Engtec Consulting Inc. | Vaughan | ON | Type II | Industrial |
| 29-May | Centre intégré universitaire de santé et de services sociaux de la Mauricie-et-du-Centre-du-Québec | Trois-Rivières | QC | Type II | Medical |
| 29-May | Centre intégré universitaire de santé et de services sociaux de la Mauricie-et-du-Centre-du-Québec | Trois-Rivières | QC | Type II | Medical |
| 29-May | St. Joseph’s Health Care, London | London | ON | Type I | Commercial |
| 3-Jun | Labo S.M. Inc. | Longueuil | QC | Type II | Industrial |
| 5-Jun | Terraprobe Testing Ltd. | Brampton | ON | Type II | Industrial |
| 6-Jun | Patriot Engineering Ltd. | Toronto | ON | Type II | Industrial |
| 6-Jun | Edward Wong & Associates Inc. | Markham | ON | Type II | Industrial |
| 7-Jun | Cott Corporation | Mississauga | ON | Type II | Industrial |
| 9-Jun | Groupe ABS Inc. | Blainville | QC | Type II | Industrial |
| 10-Jun | Labo S.M. Inc. | Longueuil | QC | Type II | Industrial |
| 10-Jun | Groupe ABS Inc. | Montréal | QC | Type II | Industrial |
| 12-Jun | Sintra Inc. | St-Charles | QC | Type II | Industrial |
| 12-Jun | Sintra Inc. | St-Charles | QC | Type II | Industrial |
| 12-Jun | Inspectrum Testing Inc. | Grande Prairie | AB | Type II | Industrial |
| 12-Jun | Sartrex Power Control Systems Inc. | Concord | ON | Type II | Commercial |
| 12-Jun | Sartrex Power Control Systems Inc. | Concord | ON | Type II | Commercial |
| 12-Jun | Sartrex Power Control Systems Inc. | Concord | ON | Type II | Commercial |
| 12-Jun | TechSpec NDT Limited | Grande Prairie | AB | Type II | Industrial |
| 12-Jun | Protekna Services Techniques Inc. | Sherbrooke | QC | Type II | Industrial |
| 13-Jun | KMH Cardiology Centres Incorporated | Mississauga | ON | Type II | Commercial |
| 13-Jun | Pavages Maska Inc. | Magog | QC | Type II | Industrial |
| 13-Jun | Labo S.M. Inc. | Sherbrooke | QC | Type II | Industrial |
| 13-Jun | Brody Inspection Ltd. | Valleyview | AB | Type II | Industrial |
| 13-Jun | Parkland Geotechnical Consulting Ltd. | Grande Prairie | AB | Type II | Industrial |
| 13-Jun | Protekna Services Techniques Inc. | Sherbrooke | QC | Type II | Industrial |
| 13-Jun | EXP Services Inc. / Les Services EXP Inc. | Sherbrooke | QC | Type II | Industrial |
| 14-Jun | Galey Inspection Services Ltd. | Sexsmith | AB | Type II | Industrial |
| 14-Jun | Aurora Inspection Limited | Sexsmith | AB | Type II | Industrial |
| 14-Jun | Step Energy Services Ltd. | Lacombe | AB | Type II | Industrial |
| 15-Jun | Graham Bros. Construction Limited | Brampton | ON | Type II | Industrial |
| 15-Jun | 20/20 ND Technology Inc. | Grande Prairie | AB | Type II | Industrial |
| 15-Jun | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Scarborough | ON | Type II | Industrial |
| 16-Jun | Bio-Rad Laboratories (Canada) Ltd. | Mississauga | ON | Type II | Commercial |
| 17-Jun | Labo S.M. Inc. | Laval | QC | Type II | Industrial |
| 19-Jun | Qualité N.D.E. Limitée | Mercier | QC | Type II | Commercial |
| 19-Jun | Qualité N.D.E. Limitée | Mercier | QC | Type II | Commercial |
| 19-Jun | Qualité N.D.E. Limitée | Mercier | QC | Type II | Commercial |
| 19-Jun | WAV Inspection Ltd. | Brooks | AB | Type II | Industrial |
| 19-Jun | Centre intégré de santé et de services sociaux de l’Outaouais | Gatineau | QC | Type I | Medical |
| 19-Jun | Centre intégré de santé et de services sociaux de l’Outaouais | Gatineau | QC | Type II | Medical |
| 20-Jun | Vision Integrity Engineering Ltd. | Brooks | AB | Type II | Industrial |
| 21-Jun | GEM Testing Ltd. | Dunmore | AB | Type II | Industrial |
| 21-Jun | AR Geotechnical Engineering Ltd. | Medicine Hat | AB | Type II | Industrial |
| 22-Jun | CEGEP de Chicoutimi | Chicoutimi | QC | Type II | Industrial |
| 22-Jun | Iron Horse Coiled Tubing Inc. | Redcliff | AB | Type II | Industrial |
| 22-Jun | Porocel of Canada, Ltd. | Medicine Hat | AB | Type II | Industrial |
| 23-Jun | Greenvac Inc. | Toronto | ON | Type II | Industrial |
| 26-Jun | Manitoba Infrastructure | West St. Paul | MB | Type II | Industrial |
| 26-Jun | Golder Associates Ltd. | Sudbury | ON | Type II | Industrial |
| 26-Jun | Pioneer Construction Inc. | Thunder Bay | ON | Type II | Industrial |
| 26-Jun | Pioneer Construction Inc. | Thunder Bay | ON | Type II | Industrial |
| 26-Jun | Teranorth Construction & Engineering Limited | Nipigon | ON | Type II | Industrial |
| 26-Jun | Taranis Contracting Group Ltd. | Thunder Bay | ON | Type II | Industrial |
| 26-Jun | Hatch Ltd. | Nipigon | ON | Type II | Industrial |
| 27-Jun | Golder Associates Ltd. | Hemlo | ON | Type II | Industrial |
| 27-Jun | Williams Operating Corporation | Marathon | ON | Type II | Industrial |
| 27-Jun | Pro-Test Professional Testing & Inspection Co. Ltd. | Winnipeg | MB | Type II | Industrial |
| 27-Jun | Pioneer Construction Inc. | Thunder Bay | ON | Type II | Industrial |
| 27-Jun | Sacopan Inc. | Sacré-Coeur-Saguenay | QC | Type II | Industrial |
| 27-Jun | Michel Lacroix Construction Inc. | Maniwaki | QC | Type II | Industrial |
| 27-Jun | Flatiron Construction Canada Limited | East St. Paul | MB | Type II | Industrial |
| 27-Jun | AV Terrace Bay Inc. | Terrace Bay | ON | Type II | Industrial |
| 27-Jun | GHD Consultants Ltd. | Chicoutimi | QC | Type II | Industrial |
| 28-Jun | Inter-Cité Construction Limitée | Chicoutimi | QC | Type II | Industrial |
| 28-Jun | College of the North Atlantic | Port aux Basques | NL | Type II | Industrial |
| 28-Jun | Canadian Blood Services/ Société canadienne du sang | Winnipeg | MB | Type II | Medical |
| 28-Jun | Englobe Corp. | La Baie | QC | Type II | Industrial |
| 28-Jun | Lac des Iles Mines Ltd. | Thunder Bay | ON | Type II | Industrial |
| 28-Jun | Canfor Pulp Ltd. | Prince George | BC | Type II | Industrial |
| 28-Jun | Canadian Forest Products Ltd. | Prince George | BC | Type II | Industrial |
| 28-Jun | Stantec Consulting Ltd. | Corner Brook | NL | Type II | Industrial |
| 28-Jun | SNC-Lavalin GEM Québec Inc. | Jonquière | QC | Type II | Industrial |
| 28-Jun | SNC-Lavalin GEM Québec Inc. | Jonquière | QC | Type II | Industrial |
| 28-Jun | Centre intégré de santé et de services sociaux de l’Outaouais | Gatineau | QC | Type II | Medical |
| 28-Jun | Natural Resources Canada | Ottawa | ON | Type II | Industrial |
| 28-Jun | Fisheries and Oceans Canada | Winnipeg | MB | Type II | Academic and research |
| 29-Jun | Corner Brook Pulp and Paper Limited | Corner Brook | NL | Type II | Industrial |
| 29-Jun | Raytheon Canada Limited / Raytheon Canada Limitée | Renfrew | ON | Type II | Commercial |
| 29-Jun | Raytheon Canada Limited / Raytheon Canada Limitée | Petawawa | ON | Type II | Commercial |
| 29-Jun | Western Memorial Regional Hospital | Corner Brook | NL | Type II | Medical |
| 29-Jun | Western Memorial Regional Hospital | Corner Brook | NL | Type II | Medical |
| 29-Jun | B. J. Halow & Son Constructors Ltd. | Rosslyn | ON | Type II | Industrial |
| 29-Jun | Bonnechere Excavating Inc. | Renfrew | ON | Type II | Industrial |
| 29-Jun | PHA Engineering Ltd. | Thunder Bay | ON | Type II | Industrial |
| 29-Jun | Bare Contracting Services Ltd. | Clarington | ON | Type II | Industrial |
| 29-Jun | Imperial Metals Corporation | Likely | BC | Type II | Industrial |
| 29-Jun | Cantex-Okanagan Construction Ltd. | Williams Lake | BC | Type II | Industrial |
| 29-Jun | New Gold Canada Inc. | Emo | ON | Type II | Industrial |
| 30-Jun | University of Northern British Columbia | Prince George | BC | Type II | Academic and research |
| 30-Jun | University of Northern British Columbia | Prince George | BC | Type II | Academic and research |
| 30-Jun | University of Northern British Columbia | Prince George | BC | Type II | Academic and research |
| 30-Jun | McElhanney Consulting Services Ltd. | Prince George | BC | Type II | Industrial |
| 30-Jun | DWB Consulting Services Ltd. | Prince George | BC | Type II | Industrial |
| 6-Jul | ROHI Engineering Ltd. | Ponoka | AB | Type II | Industrial |
| 7-Jul | WSP Canada Inc. | Calgary | AB | Type II | Industrial |
| 7-Jul | Buffalo Inspection Services (2005) Inc. | Blackfalds | AB | Type II | Industrial |
| 10-Jul | Insight Medical Holdings Ltd. | Fort McMurray | AB | Type II | Medical |
| 10-Jul | Toronto Inspection Ltd. | Markham | ON | Type II | Industrial |
| 10-Jul | Mistras Canada, Inc. | Fort McMurray | AB | Type II | Industrial |
| 10-Jul | Pembina Pipeline Corporation | Whitecourt | AB | Type II | Industrial |
| 10-Jul | Pembina Pipeline Corporation | Whitecourt | AB | Type II | Industrial |
| 10-Jul | Pembina Pipeline Corporation | Glenevis | AB | Type II | Industrial |
| 11-Jul | RTD Quality Services Inc. | Fort McMurray | AB | Type II | Industrial |
| 11-Jul | DST Consulting Engineers Inc. | Waterloo | ON | Type II | Industrial |
| 11-Jul | MNA Engineering Ltd. | Toronto | ON | Type II | Industrial |
| 11-Jul | TISI Canada Inc. | Edmonton | AB | Type II | Industrial |
| 11-Jul | Groupe ABS Inc. | Churchill Falls | NL | Type II | Industrial |
| 11-Jul | Groupe ABS Inc. | Churchill Falls | NL | Type II | Industrial |
| 11-Jul | Suncor Energy Inc. | Fort McMurray | AB | Type II | Industrial |
| 11-Jul | 1583023 Alberta Ltd. | Slave Lake | AB | Type II | Industrial |
| 11-Jul | Brion Energy Corporation | Fort MacKay | AB | Type II | Industrial |
| 11-Jul | EXP Services Inc. / Les Services EXP Inc. | Goose Bay | NL | Type II | Industrial |
| 12-Jul | BAKOSNDT Ltd. | Whitecourt | AB | Type II | Industrial |
| 12-Jul | Tracerco Radioactive Diagnostic Services Canada, Inc. | Edmonton | AB | Type II | Commercial |
| 12-Jul | Metalcare Group Inc. | Fort McMurray | AB | Type II | Industrial |
| 12-Jul | Metalcare Group Inc. | Fort McMurray | AB | Type II | Industrial |
| 12-Jul | Metalcare Group Inc. | Fort McMurray | AB | Type II | Industrial |
| 12-Jul | 1583023 Alberta Ltd. | Whitecourt | AB | Type II | Industrial |
| 12-Jul | 1583023 Alberta Ltd. | Whitecourt | AB | Type II | Industrial |
| 12-Jul | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Happy Valley-Goose Bay | NL | Type II | Industrial |
| 12-Jul | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Muskrat Falls | NL | Type II | Industrial |
| 12-Jul | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Muskrat Falls | NL | Type II | Industrial |
| 12-Jul | Ceda General Partners Ltd. | Fort McMurray | AB | Type II | Industrial |
| 12-Jul | Parkland Geotechnical Consulting Ltd. | Lethbridge | AB | Type II | Industrial |
| 12-Jul | BDT Engineering Ltd | Lethbridge | AB | Type II | Industrial |
| 13-Jul | Thurber Engineering Ltd. | Fort McMurray | AB | Type II | Industrial |
| 13-Jul | Breton N.D. Testing Incorporated | Fort McMurray | AB | Type II | Industrial |
| 13-Jul | LAW Inspection Services Inc. | Lethbridge | AB | Type II | Industrial |
| 13-Jul | Vale Newfoundland & Labrador Limited | Nain | NL | Type II | Industrial |
| 14-Jul | Alberta Agriculture and Rural Development | Lethbridge | AB | Type II | Industrial |
| 14-Jul | Stantec Consulting Ltd. | Waterloo | ON | Type II | Industrial |
| 14-Jul | Orbit Engineering Limited | Brampton | ON | Type II | Industrial |
| 17-Jul | Tembec Enterprises Inc./ Les Entreprises Tembec Inc. | Kapuskasing | ON | Type II | Industrial |
| 17-Jul | Terraprobe Testing Ltd. | Stoney Creek | ON | Type II | Industrial |
| 17-Jul | Northriver Testing Ltd. | Mission | BC | Type II | Industrial |
| 17-Jul | C. B. Engineering Ltd. | Calgary | AB | Type II | Commercial |
| 18-Jul | C. Villeneuve Construction Co. Ltd. | Hearst | ON | Type II | Industrial |
| 18-Jul | Knight Piésold Ltd. | Vancouver | BC | Type II | Industrial |
| 18-Jul | Acuren Inc. | Burnaby | BC | Type II | Industrial |
| 18-Jul | Acuren Inc. | Burnaby | BC | Type II | Industrial |
| 18-Jul | Morin Construction Ltd. | Hearst | ON | Type II | Industrial |
| 19-Jul | Fraser Valley Engineering Ltd. | Abbotsford | BC | Type II | Industrial |
| 19-Jul | Glencore Canada Corporation | Timmins | ON | Type II | Industrial |
| 19-Jul | GHD Consultants Ltd. | Mississauga | ON | Type II | Industrial |
| 19-Jul | 1068648 B.C. Ltd. | Abbotsford | BC | Type II | Industrial |
| 20-Jul | Kirkland Lake Gold Inc. | Matheson | ON | Type II | Industrial |
| 20-Jul | Jim Dent Construction Ltd. | Narrows Inlet | BC | Type II | Industrial |
| 20-Jul | Glencore Canada Corporation | Timmins | ON | Type II | Industrial |
| 20-Jul | Terratech Solutions Ltd. | Calgary | AB | Type II | Industrial |
| 24-Jul | Diavik Diamond Mines (2012) Inc. | Yellowknife | NT | Type II | Industrial |
| 24-Jul | Diavik Diamond Mines Inc. | Yellowknife | NT | Type II | Industrial |
| 24-Jul | Saskatchewan Cancer Agency | Regina | SK | Type II | Medical |
| 24-Jul | Hudbay Minerals Inc | Flin Flon | MB | Type II | Industrial |
| 24-Jul | Hudbay Minerals Inc | Flin Flon | MB | Type II | Industrial |
| 25-Jul | Manitoba Infrastructure | Thompson | MB | Type II | Industrial |
| 25-Jul | Manitoba Infrastructure | Thompson | MB | Type II | Industrial |
| 26-Jul | Kontzamanis, Graumann, Smith MacMillan Inc. | Leaf Rapids | MB | Type II | Industrial |
| 26-Jul | Saskatchewan Cancer Agency | Saskatoon | SK | Type II | Medical |
| 27-Jul | Manitoba Hydro | Split Lake | MB | Type II | Industrial |
| 27-Jul | Groupe ABS Inc. | Montréal | QC | Type II | Industrial |
| 27-Jul | Stantec Consulting Ltd. | Gillam | MB | Type II | Industrial |
| 28-Jul | M.J. Davenport & Associates Ltd. | Otonabee | ON | Type II | Industrial |
| 28-Jul | Uni-Vert Tech Inc. | Sainte-Marcelline de Kildare | QC | Type II | Commercial |
| 31-Jul | Mosaic Canada ULC | Belle Plaine | SK | Type II | Industrial |
| 31-Jul | University of New Brunswick | Fredericton | NB | Type II | Academic and research |
| 31-Jul | University of New Brunswick | Fredericton | NB | Type II | Academic and research |
| 31-Jul | University of New Brunswick | Fredericton | NB | Type II | Academic and research |
| 31-Jul | University of New Brunswick | Fredericton | NB | Type II | Academic and research |
| 31-Jul | Clifton Associates Ltd. | Regina | SK | Type II | Industrial |
| 31-Jul | Clifton Associates Ltd. | Regina | SK | Type II | Industrial |
| 31-Jul | Clifton Associates Ltd. | Regina | SK | Type II | Industrial |
| 31-Jul | Clifton Associates Ltd. | Regina | SK | Type II | Industrial |
| 31-Jul | Clifton Associates Ltd. | Regina | SK | Type II | Industrial |
| 31-Jul | Knight Vision Inspections Inc. | Regina | SK | Type II | Industrial |
| 31-Jul | 1068648 B.C. Ltd. | Terrace | BC | Type II | Industrial |
| 1-Aug | AM Inspection Limited | Forget | SK | Type II | Industrial |
| 1-Aug | Irving Paper | Saint John | NB | Type II | Industrial |
| 1-Aug | Pine Environmental Services Inc. | Mississauga | ON | Type II | Commercial |
| 1-Aug | WSP Canada Inc. | Estevan | SK | Type II | Industrial |
| 1-Aug | Custom Fabricators & Machinists Limited / Fabricants et Mach | Saint John | NB | Type II | Industrial |
| 1-Aug | Parkland Geotechnical Consulting Ltd. | Estevan | SK | Type II | Industrial |
| 1-Aug | Parkland Geotechnical Consulting Ltd. | Estevan | SK | Type II | Industrial |
| 1-Aug | Pretium Resources Inc. | Stewart | BC | Type II | Industrial |
| 2-Aug | WSP Canada Inc. | Estevan | SK | Type II | Industrial |
| 2-Aug | Port City Inspection Services Ltd. | Saint John | NB | Type II | Industrial |
| 2-Aug | Red Chris Development Company Ltd. | Iskut | BC | Type II | Industrial |
| 2-Aug | Buffalo Inspection Services (2005) Inc. | Estevan | SK | Type II | Industrial |
| 3-Aug | PEI Department of Transportation, Infrastructure and Energy | Mount Stewart | PE | Type II | Industrial |
| 3-Aug | AM Inspection Limited | Kenosee Lake | SK | Type II | Industrial |
| 3-Aug | Almadon Holdings Ltd. | Calgary | AB | Type II | Medical |
| 3-Aug | Fundy Engineering & Consulting Limited | Clyde River | PE | Type II | Industrial |
| 3-Aug | EastTech Engineering Consultants Inc. | Mount Stewart | PE | Type II | Industrial |
| 8-Aug | R.M. Belanger Limited | Chelmsford | ON | Type II | Industrial |
| 8-Aug | Dr. Melanie Dara Hobbs, P. Eng | Coldbrook | NS | Type II | Industrial |
| 9-Aug | E.F. Monk Holdings Limited | Dartmouth | NS | Type II | Industrial |
| 9-Aug | R.M. Belanger Limited | Chelmsford | ON | Type II | Industrial |
| 9-Aug | Englobe Corp. | Dartmouth | NS | Type II | Industrial |
| 9-Aug | Teranorth Construction & Engineering Limited | Sudbury | ON | Type II | Industrial |
| 9-Aug | Best Theratronics Ltd. | Ottawa | ON | Type II | Commercial |
| 10-Aug | Nova Scotia Power Incorporated | Point Tupper | NS | Type II | Industrial |
| 10-Aug | FNX Mining Company Inc. | Levack | ON | Type II | Industrial |
| 10-Aug | 2376440 Ontario Inc. | Sudbury | ON | Type II | Medical |
| 10-Aug | Atlas Testing Labs & Services (Nova Scotia) Ltd. | Salt Springs | NS | Type II | Industrial |
| 11-Aug | Nova Scotia Power Incorporated | Point Aconi | NS | Type II | Industrial |
| 11-Aug | ALSTOM Power Canada Inc. | Lingan | NS | Type II | Industrial |
| 11-Aug | Acuren Inc. | Sudbury | ON | Type II | Industrial |
| 11-Aug | Denis Gratton Construction | Chelmsford | ON | Type II | Industrial |
| 11-Aug | Alberta Health Services | Edmonton | AB | Type II | Medical |
| 11-Aug | Elekta Inc. | Edmonton | AB | Type II | Commercial |
| 14-Aug | DeBeers Canada Inc. | Yellowknife | NT | Type II | Industrial |
| 14-Aug | De Beers Canada Inc. | Yellowknife | NT | Type II | Industrial |
| 15-Aug | IMS Systems, Inc. | Toronto | ON | Type II | Commercial |
| 15-Aug | Algoma Tubes Inc. | Sault Ste Marie | ON | Type II | Industrial |
| 17-Aug | SNC-Lavalin GEM Québec Inc. | Laval | QC | Type II | Industrial |
| 17-Aug | SNC-Lavalin GEM Québec Inc. | Longueuil | QC | Type II | Industrial |
| 17-Aug | GHD Consultants Ltd. | Saint-Laurent | QC | Type II | Industrial |
| 23-Aug | Les Laboratoires d’Essais Mequaltech Inc. | Montréal | QC | Type II | Industrial |
| 23-Aug | Les Laboratoires d’Essais Mequaltech Inc. | Montréal | QC | Type II | Industrial |
| 25-Aug | Englobe Corp. | Laval | QC | Type II | Industrial |
| 25-Aug | Mistras Services Inc. | Oakville | ON | Type II | Industrial |
| 28-Aug | Sintra Inc. | Lévis | QC | Type II | Industrial |
| 28-Aug | Le Groupe Roy Consultants Ltee | Bathurst | NB | Type II | Industrial |
| 28-Aug | GHD Consultants Ltd. | St-Romuald | QC | Type II | Industrial |
| 28-Aug | EXP Services Inc. / Les Services EXP Inc. | Lévis | QC | Type II | Industrial |
| 29-Aug | Les Laboratoires d’Essais Mequaltech Inc. | Lévis | QC | Type II | Industrial |
| 29-Aug | Location Océan Inc. | Type II | Industrial | ||
| 29-Aug | New Brunswick Power Generation Corporation | Belledune | NB | Type II | Industrial |
| 29-Aug | SNC-Lavalin GEM Québec Inc. | Montréal | QC | Type II | Industrial |
| 29-Aug | SNC-Lavalin GEM Québec Inc. | Montréal | QC | Type II | Industrial |
| 29-Aug | AV Group NB Inc. | Atholville | NB | Type II | Industrial |
| 30-Aug | SGS Canada Inc. | Montréal | QC | Type II | Industrial |
| 30-Aug | Structural Inspections Limited | Milton | ON | Type II | Industrial |
| 30-Aug | Englobe Corp. | Québec | QC | Type II | Industrial |
| 30-Aug | Les entreprises Rolland inc. | Lévis | QC | Type II | Industrial |
| 30-Aug | Mistras Services Inc. | Terrebonne | QC | Type II | Industrial |
| 30-Aug | Ciment McInnis inc. / McInnis Cement inc. | Port-Daniel-Gascons | QC | Type II | Industrial |
| 30-Aug | Laboratoires d’Expertises de Québec Ltée | Québec | QC | Type II | Industrial |
| 30-Aug | Entreprise Gestion Indorama Inc. | Montréal | QC | Type II | Industrial |
| 31-Aug | Inter-Cité Construction Limitée | Chicoutimi | QC | Type II | Industrial |
| 31-Aug | Englobe Corp. | Québec | QC | Type II | Industrial |
| 31-Aug | Englobe Corp. | Québec | QC | Type II | Industrial |
| 31-Aug | D. Crupi & Sons Limited | Toronto | ON | Type II | Industrial |
| 31-Aug | Clinique Radiologique de la Capitale Inc. | Québec | QC | Type II | Medical |
| 31-Aug | Acuren Inc. | Brossard | QC | Type II | Industrial |
| 31-Aug | SAFFA Engineering Inc. | Markham | ON | Type II | Industrial |
| 31-Aug | Laboratoires d’Expertises de Québec Ltée | Québec | QC | Type II | Industrial |
| 1-Sep | Centre hospitalier universitaire de Montréal | Montréal | QC | Type II | Medical |
| 1-Sep | Centre hospitalier universitaire de Montréal | Montréal | QC | Type II | Medical |
| 1-Sep | Inter-Cité Construction Limitée | Chicoutimi | QC | Type II | Industrial |
| 1-Sep | P. & B. Entreprises Ltée | Hâvre-Aux-Maisons | QC | Type II | Industrial |
| 1-Sep | Pavage Centre Sud du Québec Inc. | Thetford-Mines | QC | Type II | Industrial |
| 1-Sep | Laboratoires d’Expertises de Québec Ltée | Québec | QC | Type II | Industrial |
| 8-Sep | Dawson Construction Limited | Fernie | BC | Type II | Industrial |
| 11-Sep | Government of Yukon | Whitehorse | YT | Type II | Industrial |
| 11-Sep | Coco Paving Inc. | Toronto | ON | Type II | Industrial |
| 11-Sep | Coco Paving Inc. | Oshawa | ON | Type II | Industrial |
| 11-Sep | 42256 Yukon Inc. | Whitehorse | YT | Type II | Industrial |
| 11-Sep | British Columbia Cancer Agency | Victoria | BC | Type I | Medical |
| 11-Sep | British Columbia Cancer Agency | Victoria | BC | Type II | Medical |
| 12-Sep | Government of Yukon | YT | Type II | Industrial | |
| 12-Sep | Englobe Corp. | Anjou | QC | Type II | Industrial |
| 12-Sep | Coco Paving Inc. | Windsor | ON | Type II | Industrial |
| 13-Sep | Windsor Regional Hospital | Windsor | ON | Type II | Medical |
| 13-Sep | Windsor Regional Hospital | Windsor | ON | Type II | Medical |
| 13-Sep | Windsor Regional Hospital | Windsor | ON | Type II | Commercial |
| 13-Sep | Windsor Regional Hospital | Windsor | ON | Type II | Medical |
| 13-Sep | Windsor Regional Hospital | Windsor | ON | Type II | Medical |
| 13-Sep | Government of Yukon | YT | Type II | Industrial | |
| 13-Sep | Englobe Corp. | Laval | QC | Type II | Industrial |
| 13-Sep | Chilkoot Geological Engineers Ltd. | Whitehorse | YT | Type II | Industrial |
| 13-Sep | Groupe Conseil SCT inc. | Brossard | QC | Type II | Industrial |
| 13-Sep | Rampure Radiology Associates Inc. | Windsor | ON | Type II | Medical |
| 14-Sep | British Columbia Cancer Agency | Vancouver | ON | Type II | Medical |
| 14-Sep | Minto Explorations Ltd. | Pelly Crossing | YT | Type II | Industrial |
| 14-Sep | Coco Paving Inc. | Windsor | ON | Type II | Industrial |
| 14-Sep | SGS Canada Inc. | Montréal | QC | Type II | Industrial |
| 18-Sep | Artech Consulting Ltd. | Cranbrook | BC | Type II | Industrial |
| 18-Sep | GHD Consultants Ltd. | Mississauga | ON | Type II | Industrial |
| 19-Sep | Miller Paving Limited | Whitby | ON | Type II | Industrial |
| 19-Sep | Glacier Technical Services Ltd. | Cranbrook | BC | Type II | Industrial |
| 19-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 19-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 19-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 19-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 19-Sep | Bare Contracting Services Ltd. | Mississauga | ON | Type II | Industrial |
| 19-Sep | Teck Coal Limited | Elkford | BC | Type II | Industrial |
| 19-Sep | Amec Foster Wheeler Americas Limited / Amec Foster Wheeler A | Scarborough | ON | Type II | Industrial |
| 20-Sep | Englobe Corp. | Joliette | QC | Type II | Industrial |
| 20-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 20-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 20-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 20-Sep | Solmatech Inc. | Le Gardeur | QC | Type II | Industrial |
| 20-Sep | Groupe TNT Inc. / TNT Group Inc. | Laval | QC | Type II | Industrial |
| 20-Sep | Max Helmer Construction Ltd. | Invermere | BC | Type II | Industrial |
| 26-Sep | Glencore Canada Corporation | Onaping | ON | Type II | Industrial |
| 26-Sep | Acuren Inc. | Sudbury | ON | Type II | Industrial |
| 27-Sep | Coco Paving Inc. | Hamilton | ON | Type II | Industrial |
| 28-Sep | Dixie X-Ray Associates Limited | Woodbridge | ON | Type II | Medical |
| 28-Sep | Stelco Inc. | Nanticoke | ON | Type II | Industrial |
| 28-Sep | WSP Canada Inc. | Hamilton | ON | Type II | Industrial |
| 2-Oct | Centre Intégré de Santé et de Sercices Sociaux de Laval | Laval | QC | Type II | Medical |
| 2-Oct | Centre Intégré de Santé et de Sercices Sociaux de Laval | Laval | QC | Type II | Medical |
| 2-Oct | Mistras Canada, Inc. | Edmonton | AB | Type II | Industrial |
| 2-Oct | Seymour Pacific Developments Ltd. | Penticton | BC | Type II | Industrial |
| 3-Oct | University of Alberta | Edmonton | AB | Type II | Commercial |
| 3-Oct | Kelowna General Hospital | Kelowna | BC | Type II | Medical |
| 3-Oct | Kelowna General Hospital | Kelowna | BC | Type II | Medical |
| 3-Oct | All Can Inspection Services (2011) Inc. | Edmonton | AB | Type II | Industrial |
| 3-Oct | Atomic Inspection Services Ltd. | Fort St. John | BC | Type II | Industrial |
| 3-Oct | SNC-Lavalin Inc. | Fort St. John | BC | Type II | Industrial |
| 3-Oct | Ecora Engineering Ltd. | Penticton | BC | Type II | Industrial |
| 3-Oct | Deka Inspection Services Ltd. | Charlie Lake | BC | Type II | Industrial |
| 4-Oct | IRISNDT Corp. | Edmonton | AB | Type II | Industrial |
| 4-Oct | Core Laboratories Canada Ltd. | Fort St. John | BC | Type II | Industrial |
| 4-Oct | Recon Petrotechnologies Ltd. | Edmonton | AB | Type II | Industrial |
| 4-Oct | Nortech Advanced N.D.T. Ltd. | Fort St. John | BC | Type II | Industrial |
| 4-Oct | Arthon Industries Limited | Kelowna | BC | Type II | Industrial |
| 4-Oct | Acuren Inc. | Armstrong | BC | Type II | Industrial |
| 4-Oct | 1068648 B.C. Ltd. | Fort St. John | BC | Type II | Industrial |
| 4-Oct | 1068648 B.C. Ltd. | Fort St. John | BC | Type II | Industrial |
| 4-Oct | Acciona Infrastructure Canada Inc. | Fort St. John | BC | Type II | Industrial |
| 4-Oct | Acciona Infrastructure Canada Inc. | Fort St. John | BC | Type II | Industrial |
| 5-Oct | Westcoast Energy Inc. | Wonowon | BC | Type II | Industrial |
| 5-Oct | Westcoast Energy Inc. | Pink Mountain | BC | Type II | Industrial |
| 5-Oct | Westcoast Energy Inc. | Pink Mountain | BC | Type II | Industrial |
| 5-Oct | Canadian Blood Services/ Société canadienne du sang | Edmonton | AB | Type II | Medical |
| 5-Oct | William Osler Health Centre | Brampton | ON | Type II | Medical |
| 5-Oct | William Osler Health Centre | Brampton | ON | Type II | Medical |
| 5-Oct | UTQUALITY INC. | Edmonton | AB | Type II | Industrial |
| 5-Oct | Ecora Engineering Ltd. | Kelowna | BC | Type II | Industrial |
| 6-Oct | Bonnett’s Energy Services Ltd. | Red Deer | AB | Type II | Industrial |
| 6-Oct | Cantex-Okanagan Construction Ltd. | Penticton | BC | Type II | Industrial |
| 6-Oct | Montreal Neurological Institute and Hospital | Montréal | QC | Type II | Commercial |
| 10-Oct | Wayne Hall Construction Inc. | Parry Sound | ON | Type II | Industrial |
| 10-Oct | Candu Energy Inc. | Whitby | ON | Type II | Waste Nuclear Substance |
| 11-Oct | Thunder Bay Regional Health Sciences Centre | Thunder Bay | ON | Type II | Medical |
| 11-Oct | Candu Energy Inc. | Mississauga | ON | Type II | Waste Nuclear Substance |
| 11-Oct | Parkway Nuclear Services Ltd. | Lindsay | ON | Type II | Medical |
| 11-Oct | Miller Paving Limited | Wainfleet | ON | Type II | Industrial |
| 11-Oct | Knight Piésold Ltd. | North Bay | ON | Type II | Industrial |
| 11-Oct | Bare Contracting Services Ltd. | North Bay | ON | Type II | Industrial |
| 11-Oct | KDT Consulting Services | St. Charles | ON | Type II | Industrial |
| 12-Oct | Alamos Gold Inc. | Matachewan | ON | Type II | Industrial |
| 12-Oct | Mississauga Metals & Alloys | Brantford | ON | Type II | Waste Nuclear Substance |
| 12-Oct | Thunder Bay Regional Health Sciences Centre | Thunder Bay | ON | Type II | Commercial |
| 12-Oct | Thunder Bay Regional Health Sciences Centre | Thunder Bay | ON | Type II | Commercial |
| 16-Oct | Tomahawk Inspection Inc. | Weyburn | SK | Type II | Industrial |
| 17-Oct | McIntosh Lalani Engineering Ltd. | Calgary | AB | Type II | Industrial |
| 17-Oct | FB Nondestructive Examination Ltd. | Moose Jaw | SK | Type II | Industrial |
| 17-Oct | TISI Canada Inc. | Paradise | NL | Type II | Industrial |
| 17-Oct | Aker Solutions Asset Integrity and Management Canada Inc. | St. John’s | NL | Type II | Industrial |
| 17-Oct | Eclipse E-Line Services Inc. | Moose Jaw | SK | Type II | Industrial |
| 17-Oct | University of Alberta | Edmonton | AB | Type II | Waste Nuclear Substance |
| 18-Oct | City of St. John’s | St. John’s | NL | Type II | Industrial |
| 18-Oct | Echo NDE Inc. | Red Deer | AB | Type II | Industrial |
| 18-Oct | Allnorth Consultants Limited | Come by Chance | NL | Type II | Industrial |
| 18-Oct | Toronto West Cardiac and Medical Imaging Centre Ltd. | North York | ON | Type II | Medical |
| 18-Oct | AR Geotechnical Engineering Ltd. | Swift Current | SK | Type II | Industrial |
| 18-Oct | K+S Potash Canada General Partnership | Dufferin | SK | Type II | Industrial |
| 19-Oct | AM Inspection Limited | Kindersley | SK | Type II | Industrial |
| 19-Oct | Slick Inspection Limited | Kindersley | SK | Type II | Industrial |
| 19-Oct | Slick Inspection Limited | Kindersley | SK | Type II | Industrial |
| 19-Oct | Suncor Energy Inc. | St. John’s | NL | Type II | Industrial |
| 19-Oct | Union Street Geotechnical Ltd. | Red Deer | AB | Type II | Industrial |
| 19-Oct | Cal Frac Well Services Ltd. | Red Deer | AB | Type II | Industrial |
| 20-Oct | Ottawa Hospital Regional Cancer Centre | Ottawa | ON | Type II | Medical |
| 20-Oct | 2540794 Ontario Inc. | Toronto | ON | Type II | Medical |
| 20-Oct | The Ottawa Hospital | Ottawa | ON | Type II | Medical |
| 23-Oct | Qualité N.D.E. Limitée | Mercier | QC | Type II | Commercial |
| 23-Oct | 860851 Alberta Ltd. | Whitecourt | AB | Type II | Industrial |
| 23-Oct | Fibrek Canada ULC | Saint-Félicien | QC | Type II | Industrial |
| 23-Oct | Greater Niagara Medical Imaging Inc. | Niagara Falls | ON | Type II | Medical |
| 24-Oct | Pavex Ltée | St-Félicien | QC | Type II | Industrial |
| 24-Oct | Tusk Inspection Services Inc. | Fox Creek | AB | Type II | Industrial |
| 24-Oct | Oshanek Inspection Services (1972) Ltd. | Fox Creek | AB | Type II | Industrial |
| 24-Oct | Englobe Corp. | St-Félicien | QC | Type II | Industrial |
| 24-Oct | A-Tech N.D.T. Limited | Whitecourt | AB | Type II | Industrial |
| 24-Oct | Groupe Conseil SCT inc. | Dolbeau-Mistassini | QC | Type II | Industrial |
| 24-Oct | Oak Ridges Medical Diagnostic Imaging Inc. | Richmond Hill | ON | Type II | Medical |
| 24-Oct | Isologic Innovative Radiopharmaceuticals Ltd. | Burlington | ON | Type II | Commercial |
| 25-Oct | Wright Instruments Limited | Mississauga | ON | Type II | Commercial |
| 25-Oct | Rio Tinto Alcan Inc. | Jonquière | QC | Type II | Industrial |
| 25-Oct | Glencore Canada Corporation | Montréal | QC | Type II | Industrial |
| 25-Oct | Centre intégré universitaire de santé et de services sociaux de | Pointe-Claire | QC | Type II | Medical |
| 25-Oct | Pembina Pipeline Corporation | Fox Creek | AB | Type II | Industrial |
| 26-Oct | Englobe Corp. | Brantford | ON | Type II | Industrial |
| 26-Oct | Englobe Corp. | Brantford | ON | Type II | Industrial |
| 26-Oct | EnergySolutions Canada Corporation | Brampton | ON | Type II | Academic and research |
| 26-Oct | EnergySolutions Canada Corporation | Brampton | ON | Type II | Commercial |
| 26-Oct | Centre intégré universitaire de santé et de services sociaux | Chicoutimi | QC | Type II | Medical |
| 26-Oct | Centre intégré universitaire de santé et de services sociaux | Chicoutimi | QC | Type II | Medical |
| 26-Oct | Trillium Therapeutics Inc. | Mississauga | ON | Type II | Academic and research |
| 6-Nov | Civil ArSa Engineering Inc. | Queensville | ON | Type II | Industrial |
| 6-Nov | CRH Canada Inc. / Groupe CRH Canada Inc. | Mississauga | ON | Type II | Industrial |
| 6-Nov | McGill University Health Centre | Montreal | QC | Type II | Medical |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | University of Saskatchewan | Saskatoon | SK | Type II | Academic and research |
| 7-Nov | Hunt Inspection Ltd. | Stettler | AB | Type II | Industrial |
| 7-Nov | Magnum Perforating Services Inc. | Drayton Valley | AB | Type II | Industrial |
| 7-Nov | Edge Wireline Inc. | Red Deer | AB | Type II | Industrial |
| 7-Nov | Pembina Pipeline Corporation | Alsike | AB | Type II | Industrial |
| 8-Nov | Aecon Transportation West Ltd. | Calgary | AB | Type II | Industrial |
| 8-Nov | Milner Power Inc. | Grande Cache | AB | Type II | Industrial |
| 8-Nov | Titanium Tubing Technology Ltd. | Lloydminster | AB | Type II | Industrial |
| 8-Nov | Cave Inspection Ltd. | Kitscoty | AB | Type II | Industrial |
| 9-Nov | Resource Management International Inc. | Lashburn | SK | Type II | Industrial |
| 9-Nov | Solidearth Geotechnical Inc. | Lloydminster | AB | Type II | Industrial |
| 9-Nov | Prairie Mines & Royalty ULC | Edson | AB | Type II | Industrial |
| 9-Nov | Foothills Radiography & Inspection Services Ltd. | Edson | AB | Type II | Industrial |
| 9-Nov | Reliance OFS Canada Ltd. | Red Deer | AB | Type II | Industrial |
| 10-Nov | Nelson’s Welding Inspection Limited | Drayton Valley | AB | Type II | Industrial |
| 14-Nov | Boss Wireline Services Ltd. | Brooks | AB | Type II | Industrial |
| 14-Nov | Voltage Wireline Inc. | Brooks | AB | Type II | Industrial |
| 14-Nov | Jesse Garant & Associates Inc. | Windsor | ON | Type I | Industrial |
| 15-Nov | Weatherford Canada Ltd. | Dresden | ON | Type II | Industrial |
| 15-Nov | 1788966 Alberta Ltd. | Redcliff | AB | Type II | Industrial |
| 15-Nov | Saint John Regional Hospital | Saint John | NB | Type II | Medical |
| 15-Nov | Saint John Regional Hospital | Saint John | NB | Type II | Medical |
| 16-Nov | Chatham-Kent Health Alliance | Chatham | ON | Type II | Medical |
| 16-Nov | Chatham-Kent Health Alliance | Chatham | ON | Type II | Medical |
| 16-Nov | NHS - St. Catharines Site | Welland | ON | Type II | Medical |
| 16-Nov | NHS - St. Catharines Site | Welland | ON | Type II | Medical |
| 16-Nov | Greenfield Global Inc. | Chatham | ON | Type II | Industrial |
| 20-Nov | RTD Quality Services Inc. | Surrey | BC | Type II | Industrial |
| 20-Nov | CRH Canada Inc. / Groupe CRH Canada Inc. | Hamilton | ON | Type II | Industrial |
| 20-Nov | EnergySolutions Canada Corporation | Brampton | ON | Type II | Waste Nuclear Substance |
| 21-Nov | Elekta Inc. | Rimouski | QC | Type II | Commercial |
| 21-Nov | A & A Concrete X-Ray and Coring Ltd. | Surrey | BC | Type II | Industrial |
| 21-Nov | B & B Contracting (2012) Ltd. | Surrey | BC | Type II | Industrial |
| 22-Nov | Telford Geotechnical Ltd. | Kamloops | BC | Type II | Industrial |
| 22-Nov | Jim Dent Construction Ltd. | Hope | BC | Type II | Industrial |
| 22-Nov | Centre intégré de santé et de services sociaux du Bas-Saint-Laurent | Rimouski | QC | Type II | Medical |
| 22-Nov | WSP Canada Inc. | Collingwood | ON | Type II | Industrial |
| 23-Nov | Dawson Construction Limited | Kamloops | BC | Type II | Industrial |
| 23-Nov | Acuren Inc. | Kamloops | BC | Type II | Industrial |
| 23-Nov | Okanagan Material Testing and Engineering Services Ltd. | Salmon Arm | BC | Type II | Industrial |
| 23-Nov | 2539393 Ontario Inc. |
Mississauga | ON | Type II | Medical |
| 24-Nov | Stantec Consulting Ltd. |
London | ON | Type II | Industrial |
| 24-Nov |
Centre intégré de santé et de services sociaux du Bas-Saint-Laurent |
Rimouski | QC | Type I | Medical |
| 28-Nov | MyHealth Partners Inc. |
Caledon | ON | Type II | Medical |
| 5-Dec | West-Can Inspection Ltd. |
Winnipeg | MB | Type II | Industrial |
| 5-Dec | Winnipeg Regional Health Authority |
Winnipeg | MB | Type II | Commercial |
| 5-Dec | Winnipeg Regional Health Authority |
Winnipeg | MB | Type II | Commercial |
| 5-Dec | Winnipeg Regional Health Authority |
Winnipeg | MB | Type II | Academic and research |
| 6-Dec | University of Winnipeg |
Winnipeg | MB | Type II | Academic and research |
| 6-Dec | University of Winnipeg |
Winnipeg | MB | Type II | Academic and research |
| 6-Dec | DGH Engineering Ltd. |
St Andrews |
MB | Type II | Industrial |
| 6-Dec | NHS - St. Catharines Site |
Niagara Falls |
ON | Type II | Medical |
| 6-Dec | Cardiovascular Care Centre Inc. |
Mississauga | ON | Type II | Medical |
| 8-Dec | Wilfrid Laurier University |
Waterloo | ON | Type II | Academic and research |
| 8-Dec | Wilfrid Laurier University |
Waterloo | ON | Type II | Academic and research |
| 11-Dec | Northern Health Authority |
Fort St. John |
BC | Type II | Medical |
| 11-Dec | Northern Health Authority |
Fort St. John |
BC | Type II | Medical |
| 11-Dec | Rivest Technologies Incorporated |
Edmonton | AB | Type II | Industrial |
| 11-Dec | Canadian Forest Products Ltd. |
Taylor | BC | Type II | Industrial |
| 11-Dec | Radioprotection Inc. |
Val d’Or |
QC | Type II | Commercial |
| 11-Dec | SNC-Lavalin GEM Québec Inc. |
Val d’Or |
QC | Type II | Industrial |
| 11-Dec | Vancouver Coastal Health Authority |
Vancouver | BC | Type I | Medical |
| 12-Dec | The Hospital for Sick Children |
Toronto | ON | Type II | Medical |
| 12-Dec | The Hospital for Sick Children |
Toronto | ON | Type II | Medical |
| 12-Dec | The Hospital for Sick Children |
Toronto | ON | Type II | Medical |
| 12-Dec | Peace Country Technical Services Ltd. |
Dawson Creek |
BC | Type II | Industrial |
| 12-Dec | Resolute FP Canada Inc. / PF Résolu Canada Inc. |
Amos | QC | Type II | Industrial |
| 12-Dec | Plains Midstream Canada ULC |
Edmonton | AB | Type II | Industrial |
| 12-Dec | Plains Midstream Canada ULC |
Sherwood Park |
AB | Type II | Industrial |
| 12-Dec | Plains Midstream Canada ULC |
Edmonton | AB | Type II | Industrial |
| 12-Dec | Brocor Construction Ltd. |
Dawson Creek |
BC | Type II | Industrial |
| 12-Dec | Mines Abcourt Inc. / Abcourt Mines Inc. |
Amos | QC | Type II | Industrial |
| 12-Dec | Tryon Engineering Incorporated |
Dawson Creek |
BC | Type II | Industrial |
| 13-Dec | Northern Alberta Institute of Technology |
Edmonton | AB | Type II | Industrial |
| 13-Dec | Northern Alberta Institute of Technology |
Edmonton | AB | Type II | Industrial |
| 13-Dec | Construction Norascan Inc. |
Amos | QC | Type II | Industrial |
| 13-Dec | Toronto Cardiology Associates Inc. |
Toronto | ON | Type II | Medical |
| 13-Dec | Trillium Beverage Inc. |
North York |
ON | Type II | Industrial |
| 13-Dec | SNC-Lavalin Inc. |
Fort Nelson |
BC | Type II | Industrial |
| 13-Dec |
Centre intégré de santé et de services sociaux de l’Abitibi-Témiscamingue |
Val d’Or |
QC | Type II | Medical |
| 13-Dec |
Centre intégré de santé et de services sociaux de l’Abitibi-Témiscamingue |
Val d’Or |
QC | Type II | Medical |
| 14-Dec | Les Mines Agnico-Eagle Ltée / Agnico-Eagle Mines Ltd. |
Val d’or |
QC | Type II | Industrial |
| 14-Dec | Echo NDE Inc. |
Red Deer |
AB | Type II | Industrial |
| 14-Dec | Canadian Malartic GP |
Malartic | QC | Type II | Industrial |
| 14-Dec | Halliburton Canada |
Nisku | AB | Type II | Industrial |
Appendix E: Compliance rating levels
The following rating levels, as shown in table 15, reflect the transition in rating terminology used by the CNSC. While inspection reports may still use the previous rating levels, licensees that use nuclear substances and radiation devices can expect this transition to take place in time.
Table 15 : Compliance rating terminology
| Previous rating level | Description | New rating level | Description |
|---|---|---|---|
| A | Exceeds expectations | FS | Fully satisfactory |
| B | Meets expectations | SA | Satisfactory |
| C | Improvement is required | BE | Below expectations |
| D | This area is seriously compromised | ||
| E | Breakdown | UA | Unacceptable |
Fully satisfactory (FS)
Safety and control measures implemented by the licensee are highly effective. In addition, compliance with regulatory requirements is fully satisfactory, and compliance within the SCA exceeds requirements and CNSC expectations. Overall, compliance is stable or improving, and any problems or issues that arise are promptly addressed.
Satisfactory (SA)
Safety and control measures implemented by the licensee are sufficiently effective. In addition, compliance with regulatory requirements is satisfactory. Compliance within the SCA meets requirements and CNSC expectations. Any deviation is minor and any issues are considered to pose a low risk to the achievement of regulatory objectives and CNSC expectations. Appropriate improvements are planned.
Below expectations (BE)
Safety and control measures implemented by the licensee are marginally ineffective. In addition, compliance with regulatory requirements falls below expectations. Compliance within the SCA deviates from requirements or CNSC expectations to the extent that there is a moderate risk of ultimate failure to comply. Improvements are required to address identified weaknesses. The licensee is taking appropriate corrective action.
Unacceptable (UA)
Safety and control measures implemented by the licensee are significantly ineffective. In addition, compliance with regulatory requirements is unacceptable and is seriously compromised. Compliance within the SCA is significantly below requirements or CNSC expectations, or there is evidence of overall non-compliance. Without corrective action, there is a high probability that the deficiencies will lead to unreasonable risk. Issues are not being addressed effectively, no appropriate corrective measures have been taken and no alternative plan of action has been provided. Immediate action is required.
Appendix F: Grading inspections
For all inspections, Canadian Nuclear Safety Commission (CNSC) inspectors evaluate a licensee’s performance against regulatory requirements found in the Nuclear Safety and Control Act, its regulations and conditions included in the licensee’s licence. During an inspection, the inspector verifies compliance with specific regulatory requirements and assigns a grade (i.e., a compliance rating) based on that inspector’s observations. (Please refer to appendix E for information on the compliance ratings for inspection.) Each requirement is ranked according to the relative risk of the particular regulatory requirement: high, medium or low. The requirements are linked to a particular safety and control area (SCA), and each SCA has different numbers of requirements. The scope of the inspections determines which of the requirements are to be inspected. For the majority of licensees, inspection results are determined as follows:
- Inspection evidence is entered into a licensing and compliance system, which uses a complex algorithm to calculate an overall grade for each SCA based on the inspector’s grades.
- The SCA grade is based on the worst grade of the high-risk requirements. The SCA grade will be the lowest grade assigned to a high-risk requirement by an inspector, unless an unacceptable rating was assigned to a medium-risk requirement. In cases in which a medium-risk risk requirement has been assessed as unacceptable, the SCA grade will be one grade lower than the lowest grade assigned to high-risk requirement.
- If no high-risk requirements were inspected, the SCA grade equals the worst grade from the medium-risk requirements.
- If no high-risk or medium-risk requirements were inspected, no grade is assigned for that SCA. In other words, no SCA grade is assigned if the data come from low-risk requirements only.
For inspections not recorded in the licensing and compliance system, inspectors review each compliance expectation and determine the overall rating of the SCA based on the magnitude of the non-compliances.
Figure 64 shows a blank inspection worksheet used by inspectors to conduct a compliance inspection. This worksheet is specific to the use of portable gauges. Figure 65 shows criteria that may be used in inspections of accelerators and Class II facilities.
Table 16: Sample grading guidance for accelerator and Class II facilities
| SCA | Fully satisfactory (FS) | Satisfactory (SA) | Below expectations (BE) | Unacceptable (UA) | Mitigating factor | Aggravating factor | Grade | Explanation/ justification |
|---|---|---|---|---|---|---|---|---|
| Radiation protection |
Radiation doses are equal to or less than the norm for the sector. Contamination, if applicable, did not affect a worker. |
Increased dose below reportable limits. Contamination that could affect a worker. |
Exposure to a worker in excess of regulatory limits. An incident that would result in a licensee exceeding action level limits (see section 6 of the Radiation Protection Regulations). Limited contamination that could affect a few persons or limited area. |
Exposures to multiple workers in excess of regulatory limits. Widespread contamination to several persons or within a place. |
||||
| Physical design | No significant weaknesses in any element of the facility design. | Reduced redundancy that is not likely to prevent a safety-related system from meeting its design intent. |
Compromise to barriers where defence in depth would be considered reduced; however, redundancy
remains. Compromise to safety due to a situation that was not previously evaluated and is believed to be probable. |
Compromise to barriers where defence in depth would be considered inadequate. Compromise to safety due to a situation that was not previously evaluated and is believed to be probable. |
||||
| Operating performance | No significant lapses in conduct of licensed activities in accordance with licensee procedures or processes. | Partial failure to conduct licensed activities in accordance with one licensee procedure or processes. | Failure to conduct licensed activities in accordance with one or more licensee procedures and processes. | Widespread systemic failure to ensure licensed activities conducted according to licensee procedures and processes. | ||||
| Fitness for service | No significant risk that systems or components will not remain effective or that equipment will not be able perform its intended function when called upon to do so. | Partial failure to ensure single system or components remain effective or equipment is able to perform its intended function when called upon to do so. | Failure to ensure single system or components remain effective or equipment is able to perform its intended function when called upon to do so. | Widespread systemic failure to ensure systems and components remain effective and equipment is able to perform its intended function when called upon to do so. | ||||
| Security | No significant weaknesses in security. | Weaknesses in access control or barrier. | Failure in one or more barriers designed to delay access to security Category 1 or 2 sources. | Widespread systemic failure to adhere to security plan. | ||||
| Packaging and transport | No significant weaknesses in packaging and transport procedures and processes. | Failure in one of the licensee’s packaging and transport procedures and processes. | Failure in one or more elements of the licensee’s packaging and transport procedures and processes. | Widespread systemic failure to adhere to licensee’s packaging and transport procedures and processes. |
Appendix G: Relevant Regulatory References
The following are a list of regulatory references that apply to the use of nuclear substances and prescribed equipment. The list is not exhaustive.
Act and regulations
- Nuclear Safety and Control Act
- Administrative Monetary Penalties Regulations (Canadian Nuclear Safety Commission)
- Class II Nuclear Facilities and Prescribed Equipment Regulations
- eneral Nuclear Safety and Control Regulations
- Nuclear Substances and Radiation Devices Regulations
- Packaging and Transport of Nuclear Substances Regulations, 2015
- Radiation Protection Regulations
- Transportation of Dangerous Goods Regulations
Regulatory documents
- REGDOC-1.4.1, Licence Application Guide: Class II Nuclear Facilities and Prescribed Equipment (draft)
- REGDOC-1.5.1, Application Guide: Certification of Radiation Devices or Class II Prescribed Equipment
- REGDOC-1.6.1, Licence Application Guide: Nuclear Substances and Radiation Devices, Version 2
- REGDOC-2.2.3, Personnel Certification: Radiation Safety Officers
- REGDOC-2.2.3, Personnel Certification: Exposure Device Operators
- G-129, rev. 1, Keeping Radiation Exposures and Doses “As Low as Reasonably Achievable (ALARA)”
- REGDOC-2.9.1, Environmental Protection: Environmental Principles, Assessments and Protection Measures, Version 1.1
- REGDOC-2.12.3, Security of Nuclear Substances: Sealed Sources
- REGDOC-2.14.1, Information Incorporated by Reference in Canada’s Packaging and Transport of Nuclear Substances Regulations, 2015
- REGDOC-3.6, Glossary of CNSC Terminology
- Regulatory Policy P-290, Managing Radioactive Waste
Footnotes
- Footnote 1
-
Information about the categorization of sealed sources and their relative risks can be found on the CNSC website.
- Footnote 2
-
Exceptions may be made for certain lower-risk activities such as the operation of mobile industrial accelerators and oil well logging accelerators.
- Footnote 3
-
A radiopharmaceutical is a drug containing a radioactive substance that is used in medical imaging and cancer diagnostics and treatment.
Page details
- Date modified: